Diagnostic Techniques to Evaluate Obstructive or Reflexive Epiphora
Updated May 2024
Background
Patients with tearing represent a common challenge to the evaluating physician who treats lacrimal disorders, ranging from epiphora due to nasolacrimal obstruction to epiphora in the setting of a patent nasolacrimal drainage apparatus.
Careful history and physical examination techniques can help to elucidate the cause of the patient’s tearing and lead to appropriate treatment recommendations.
Disorders associated with tearing/epiphora
Problems of the lacrimal drainage (excretory) system
- Congenital nasolacrimal duct obstruction
- Lacrimal pump failure (lower eyelid laxity or ectropion; lateral canthal dystopia, paretic from Bell’s palsy)
- Punctal stenosis
- Idiopathic
- Severe blepharitis
- History of viral keratoconjunctivitis
- Ocular cicatricial pemphigoid
- Pseudo-pemphigoid associated with anti-glaucoma eyedrops
- Stevens-Johnson syndrome (toxic epidermal necrolysis)
- Allergy-induced Punctal Congestion Syndrome (Bian, 2021)
- Punctal ectropion
- Canalicular stenosis/obstruction
- History of prior punctal plug placement (especially intracanalicular plugs)
- Canaliculitis
- History of systemic chemotherapy
- Docetaxol
- Widely used agent in treatment of breast, lung, and prostate cancer
- Higher incidence of canalicular stenosis and lacrimal duct obstruction in patients receiving treatment weekly (64%) versus every 3 weeks (39%) (Esmaeli, J Clin Oncol. 2006)
- Regular surveillance by lacrimal specialist is important because timely intervention of topical corticosteroid drops or silicone bicanalicular intubation in those with progressive stenosis might avoid subsequent need for more-involved procedures such as CDCR/Jones tube placement.
- 5-FU
- Antimetabolite agent administered systemically for breast, colon, head and neck, and pancreatic cancer
- Has been associated with punctal and canalicular stenosis (about 30%) that might require bicanalicular silicone intubation and possibly CDCR/Jones tube placement (Fezza, Ophthalmic Surg Lasers. 1999)
- Autoimmune cicatrizing disease
- Ocular cicatricial pemphigoid
- Stephens-Johnson syndrome (toxic epidermal necrolysis)
- Lichen planus (in particular the more severe vulvovaginalgingival syndrome) (Webber, Arch Dermatol. 2012)
- Prior eyelid laceration with or without canalicular involvement
- Eyelid neoplasm/malignancy
- History of viral keratoconjunctivitis causing scarring/stenosis
- Common canalicular stenosis/obstruction
- Primary acquired nasolacrimal duct obstruction
- Secondary nasolacrimal duct obstruction
- Facial trauma/mid-face fractures
- Prior facial surgery, e.g. rhinoplasty with lateral osteotomy
- Prior intranasal surgery, e.g. inferior turbinate out-fracture/turbinate reduction/turbinectomy with inferior meatal scarring
- Intranasal inflammation
- Allergic rhinitis
- Granulomatosis with polyangiitis (formerly Wegeners granulomatosis)
- Sarcoidosis
- Lethal midline granuloma
- Neoplasia
- Invading facial malignancy, e.g. basal cell carcinoma, squamous cell carcinoma
- Inverted papilloma
- Lymphoma of lacrimal sac
- Intranasal (Squamous carcinoma, metastatic disease, lymphoma)
- Patients with simultaneous bilateral nasolacrimal duct obstruction can be twice as likely to have an underlying systemic condition (e.g., sarcoidosis, lichen planus, granulomatosis with polyangiitis) compared to unilateral cases (Sobel, Ophthal Plast Reconstr Surg. 2014).
- Prior radioactive iodine therapy, particularly higher-level dosing as for treatment of thyroid cancer (> 150 mCi I131)
- Preferential uptake of I131 by sodium-iodide symporter within nasolacrimal duct mucosa leading to scarring/stenosis (Morgenstern, Ophthal Plast Reconstr Surg. 2005).
Ocular surface disease–related tearing/reflexive epiphora
Dry eye syndrome
- Primary dry eye syndrome
- Sjogrens syndrome
Blepharitis
- Anterior blepharitis with eyelash scruff
- Posterior blepharitis/Meibomian dysfunction
- Rosacea blepharitis
Exposure keratopathy — lagophthalmos
- Facial nerve weakness
- Trauma
- Post-blepharoplasty
- Thyroid eye disease
- Exophthalmos
- Lagophthalmos
- Upper and lower eyelid retraction
- Eyelid malposition: ectropion/entropion
- Trichiasis
Other keratopathy
- Epithelial basement membrane dystrophy (map-dot-fingerprint)
- Pseudophakic bullous keratopathy
- Decompensated Fuchs corneal dystrophy
Secondary ocular surface irritation with reflexive tearing
- Allergic conjunctivitis
- Viral conjunctivitis
- Kerato-conjunctivitis from ophthalmic drops
- Anti-glaucoma drops
- Topical corticosteroid drops (suspension)
- Anti-viral drops
- Over-the-counter drop overuse, especially “get the red out” drops
- Homeopathic preparations
Other
- Congenital glaucoma
- Aberrant CN VII innervation/regeneration
- “Crocodile tears” release of tears from lacrimal gland with salivation or jaw movements
Diagnostic probing (palpation) and irrigation of lacrimal drainage system
Indications/contraindications
Indications
- The primary assessment of the patient with tearing and epiphora; performed to identify and characterize a potential lacrimal outflow obstruction
Possible contraindication: acute infection
- Higher risk of false passage creation in friable infected tissue
- Potentially more painful to patient
- Potential spread of infection
Exam
- External observation for epiphora (tears onto cheek)
- Assess presence, position, and patency of all four lacrimal puncta
- Punctal “pouting” or discharge (as in canaliculitis)
- Canalicular erythema
- Presence of dacryocystocele or dacryocystitis
- Elevated tear meniscus (increased tear lake)
- Assess eyelid position, tone, blink to assess “lacrimal pump” function (Figure 1)
- Lateral canthus should be slightly higher than medial canthus
- “Snap-back” test: following distraction and release lid should snap-back to globe; requirement of blink to return lid to globe indicates significant eyelid laxity
- Eyelid distraction test: lid should not be able to be distracted from globe more than 5-6mm – a larger amount indicates significant eyelid laxity
- Delayed fluorescein dye disappearance test (Figure 2)
- Assessment of ocular surface for underlying dryness or poor tear quality
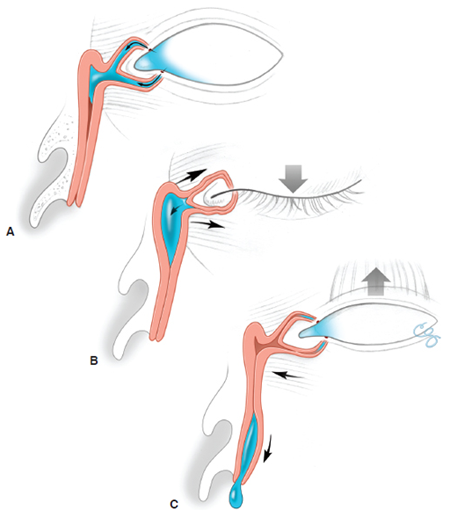
Figure 1. Lacrimal pump. (A) In the relaxed state, the puncta lie in the tear lake. (B) With eyelid closure, the orbicularis contracts. The pretarsal orbicularis squeezes and closes the canaliculi. The preseptal orbicularis, which inserts into the lacrimal sac, pulls the lacrimal sac open, creating a negative pressure that draws the tears into the sac. (C) With eyelid opening, the orbicularis relaxes, and the elastic forces create a positive pressure in the sac that propels the tears down the duct. Illustration by Christine Gralapp.
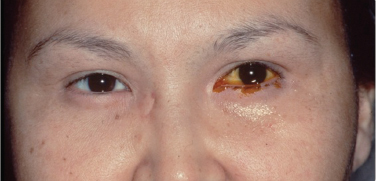
Figure 2. Dye disappearance test. Image courtesy Andrew Harrison, MD.
Basic secretion test
- Evaluates tear production with minimal ocular surface stimulation
- Topical anesthetic instilled in eye
- Conjunctival cul-de-sac dried with tissue paper
- Filter paper strip placed in inferotemporal fornix for 5 minutes
- Normal tear production > 15 mm
- Less than 10 mm indicative of relative hyposecretion: Patient’s symptoms likely to respond to artificial tear drops if applied on a regular basis (e.g. QID)
Schirmer I
- Evaluates tear production but variable response due to irritation by filter paper
- No topical anesthetic
- Dry conjunctival cul-de-sac with tissue paper
- Filter paper strip placed in inferotemporal fornix for 5 minutes
- Normal tear production greater than 15 mm
- Patients with less than 15 mm might have positive response to regular use of artificial tear drops
Schirmer II
- Evaluates potential for any tear production in patients with severe dry eyes (e.g., Sjogren syndrome)
- This test is only done in patients with a significantly decreased Schirmer 1 test
- No topical anesthetic
- Dry conjunctival cul-de-sac with tissue paper
- Filter paper strip placed in inferotemporal fornix for 5 minutes
- A cotton applicator is used to irritate the inside of the nose and stimulate tearing
- A moistened filter paper represents some ability of the main lacrimal gland to respond to a noxious stimulus
Tear breakup time
- This test is used to qualitatively assess the quantity and quality of tears produced
- Fluorescein is instilled in the affected eye
- At the slit lamp, the tear film is assessed
- Patient is asked to blink and then stare
- Initial lack of corneal tear coating with blink might indicate a general deficiency of tear production
- If initially smooth coating of cornea occurs, but breaks up after 15 seconds, this is likely normal
- If tear film breaks up in less than 10 seconds, this might represent a deficiency of mucin or lipid components of tears
Procedure alternatives
- Lacrimal irrigation without probing — although probing of upper lacrimal system is generally performed concurrently with cannula used for irrigation
- Physiologic patency of the lacrimal excretory system might include dye disappearance test and intranasal exam, either via nasal speculum and headlight or by formal nasal endoscopy.
Jones testing
- Jones 1
- Drop of fluorescein is instilled in cul-de-sac and a cotton-tipped applicator is placed into the inferior meatus.
- If dye is detected after 5 minutes, the system is patent and functioning.
- If no dye, proceed to Jones 2.
- Jones 2
- The cul-de-sac is cleared of remaining fluorescein and then lacrimal irrigation is performed.
- If dye is detected in the nose, it suggests that on initial testing (Jones 1), some dye entered the lacrimal sac, but could not proceed down the nasolacrimal duct, suggesting duct stenosis or blockage.
- If no dye is detected, it suggests no dye entered the lacrimal sac during Jones 1 and there is punctal or canalicular stenosis or a tear pump deficiency.
Specialized radiographic assessment of lacrimal drainage system
- CT scan of facial bones can be obtained in cases of suspected trauma or tumor (palpable mass, bleeding, etc.).
- Dacryocystography (DCG)
- To assess the anatomy of the lacrimal drainage system, e.g., fistula, diverticulum, stone/dacryolith, mass
- Nonphysiologic test: Radiotracer dye is injected under pressure via punctum and canaliculus followed by radiography.
- Considered when suspicion for any of the above or in some cases of “functional” nasolacrimal duct obstruction, e.g., patent system to irrigation, but symptomatic epiphora in absence of other causes.
- DCG shows greater anatomical detail of the lacrimal drainage apparatus than DSG.
- Specialized forms of DCG (CT-based and MR-based) exist, but are used less frequently.
- Dacryoscintigraphy (DSG)
- Physiological visualization of the function of the lacrimal drainage system
- Radiotracer eye drops applied followed by serial imaging to depict the natural “physiologic flow” of tracer through system
- Also reserved for “atypical” cases of epiphora/functional nasolacrimal obstruction, e.g., a patient patent to lacrimal irrigation but with dacryoscintigraphic evidence of limitation of dye flow past sac/duct junction (preductal delay) might benefit from DCR surgery.
- Presac retention (tracer does not enter the lacrimal sac) suggests a lacrimal pump or canalicular dysfunction.
- Despite differences in technique, sensitivity of detecting a lacrimal outflow apparatus abnormality is relatively similar between DCG and DSG (93% and 95%, respectively) (Wearne, Br J Ophthalmol 1999).
Instrumentation and technique of lacrimal probing and irrigation
Anesthesia
- Adults
- Topical anesthesia
- Proparacaine or tetracaine eye drops followed by cotton pledget soaked in 4% lidocaine solution applied to the area of the punctum/canaliculus for two to five minutes.
- Infants: usually general anesthesia
Technique (Figure 3)
- Lateral traction on eyelid
- Punctal dilation
- Gentle probing of punctum and canaliculus with a Bowman lacrimal probe (generally begin with smaller size probe such as “00”).
- This part of the procedure can also be performed with the cannula used for lacrimal irrigation.
- Note length of probe that can be inserted prior to resistance (“soft stop”) to measure location of a canalicular obstruction, or successful passage of probe into lacrimal sac with contact with bone (“hard stop”) indicating normal and patent canaliculus.
- When a soft-stop is encountered:
- The probe is gently withdrawn.
- Care is taken to ensure adequate lateral eyelid traction is maintained and that the probe is aimed along the correct trajectory.
- An attempt is made to pass the probe again through to a hard stop.
- Irrigation is performed with a lacrimal cannula on a 3-cc syringe with sterile saline.
- The cannula is withdrawn from the hard stop so that the tip of the cannula is intracanalicular and not all the way to the lacrimal sac.
- Gentle irrigation is then performed and an assessment made for ease of injection, reflux around the injected punctum and canaliculus, reflux from the opposite punctum (e.g. upper punctum reflux from lower punctum irrigation).
- Irrigation should also be attempted in the presence of a canalicular “soft-stop” because this maneuver will help differentiate a complete canalicular obstruction from a partial or stenosed segment.
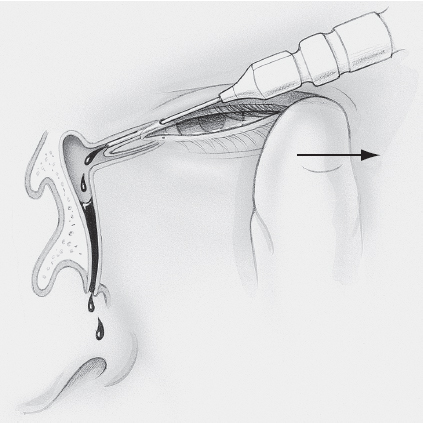
Figure 3. Lacrimal probing technique. Illustration by Christine Gralapp.
Potential complications of the procedure
- Tearing of lacrimal punctum by over-aggressive dilation — expected to heal spontaneously without sequelae
- False passage creation prevented by gentle probing technique and care to maintain the probe along the expected canalicular trajectory while maintaining lateral eyelid traction during the procedure
- Generally no treatment required because the false passage is expected to spontaneously heal
Interpretation (Figure 4)
Soft stop identifies the presence and position of canalicular obstruction.
Hard stop (lacrimal bone of lacrimal sac fossa) demonstrates a patent canaliculus/common canaliculus into the lacrimal sac.
A stenotic portion of the canaliculus can be identified and localized with gentle probing and measuring distance from punctum to end of probe at obstruction.
Irrigation of saline to the nose indicates a grossly patent system.
Resistance to irrigation with some flow of saline to nose and some reflux indicates a stenotic nasolacrimal duct.
Complete reflux from the same punctum indicates improper cannula position or canalicular/common-canalicular obstruction.
Complete reflux from opposite punctum indicates complete nasolacrimal duct obstruction if previously able to advance to a hard stop or obstruction of the common canaliculus if not able to advance fully to a hard stop.
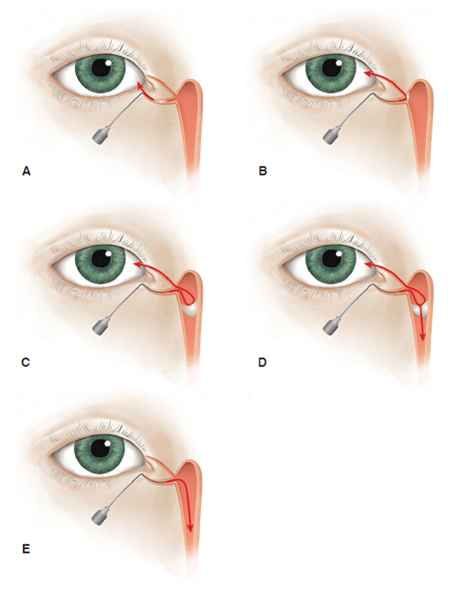
Figure 4. Interpretation of lacrimal irrigation results. Illustration by Cyndie C. H. Wooley.
Nasal endoscopy
Indications
- Used to evaluate for intranasal causes of lacrimal outflow obstruction, e.g., mass, severe rhinitis, and turbinate hypertrophy/malposition
- Used to rule out nasal septum or turbinate abnormalities that might require correction at time of lacrimal surgery such as during dacryocystorhinostomy
- Septal deviation or hypertrophy limiting access to the lacrimal sac area or high risk of contributing to postoperative adhesion formation and ostium closure (Chan, Am J Otolaryngol. 2014).
- Used to monitor status of dacryocystorhinostomy (DCR) ostium or Jones tube following lacrimal surgery
- Endoscopic dye test
- Surveillance for silicone-tube associated pyogenic granuloma formation following DCR surgery. (Figure 5)
- Facilitate repositioning or removal of silicone tubing
- Identification of other intranasal pathology
- Chronic rhinitis
- Sinusitis (pus at sinus ostia)
- Intranasal neoplasia
- Hamartoma
- Nasal polyp
- Squamous carcinoma
- Lymphoma
- Destructive process: granulomatosis with polyangiitis (Wegeners)
- Altered anatomy from prior trauma or surgery
- Inferior turbinate malposition occluding the inferior meatus
- History of lateral osteotomies with rhinoplasty surgery
Pre-procedure evaluation
- History suggestive of lacrimal outflow obstruction and/or intranasal pathology
- Clinical examination of lacrimal outflow system including pertinent external exam, slit-lamp biomicroscope examination, fluorescein dye disappearance testing, upper system probing and irrigation
Procedure alternatives
- Direct examination with nasal speculum
- Otolaryngology/rhinology consultation
- Sinus computed tomography (CT) studies (complementary)
Instrumentation and technique
- Topical vasoconstrictor/anesthetic spray, such as oxymetazoline/lidocaine atomized spray, often used
- Use of zero or 30-degree, 2.7–4.0-mm video endoscope system
- Patient reclined, examiner (right-handed) stands to right side of patient viewing screen positioned behind and to the left of the head of the patient.
- Scope gently advanced through right nostril first.
- Exam is easier if patient’s head slightly tilted toward examiner for right-side examination.
- Note is made of appearance of nasal mucosa and position of nasal septum, prominence of septal swell body, presence of septal spurs or deviation, size and position of inferior turbinate, possible evaluation of inferior meatus and region of valve of Hasner (easier with 30 degree scope and good topical anesthesia), evaluation of access and space around the maxillary line (corresponding to the suture between lacrimal bone and maxillary bone between anterior and posterior lacrimal crests, i.e. potential location of DCR ostium, middle turbinate and relation to lacrimal sac/axilla of middle turbinate. Scope then carefully withdrawn and process repeated for left nasal cavity with patient’s head rotated to neutral position.
Complications
- Inability to visualize above structures due to nasal septal deviation, mucosal edema, etc.
- Abrasion of the nasal mucosa causing epistaxis
- Patient discomfort
Interpretation of this diagnostic procedure
Interpretation of nasal endoscopy requires knowledge of intranasal anatomy (Figure 6).
- Inferior meatus beneath inferior turbinate: The nasolacrimal duct drains here via the Valve of Hasner.
- Inferior turbinate
- Middle meatus receives drainage from the frontal sinuses, anterior and middle ethmoids, and maxillary sinus (osteomeatal complex).
- Middle turbinate: The anterior root of the middle turbinate is medial to the lacrimal sac.
- Maxillary line
- A prominent linearity running cranio-caudally along the lateral nasal wall corresponding to the suture-line between maxillary and lacrimal bones.
- Important landmark for various endonasal endoscopic surgeries including endoscopic dacryocystorhinostomy
- Uncinate process: ridge-like elevation of lateral wall in middle meatus
- Superior meatus receives drainage from the posterior ethmoid air cells.
- Superior turbinate
- Sphenoethmoid recess receives drainage from the sphenoid sinus.
- Nasal septum
Visualization of intranasal abnormalities (e.g., significant septal deviation, mucosal hyperemia/discharge/ulceration, masses, synechiae) might prompt otolaryngology consultation for further evaluation and medical/surgical treatment of sinonasal abnormalities as indicated (e.g., possible septoplasty at time of DCR or prior to DCR, sinus carcinoma).
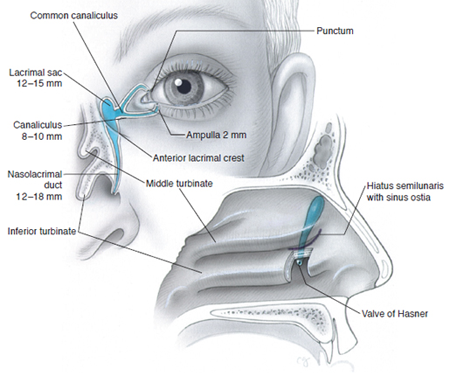
Figure 6. Normal anatomy of the lacrimal excretory system. Measurements are for adults. Illustration by Christine Gralapp.
References and additional resources
- AAO, Surgery of the Eyelid, Orbit & Lacrimal system, Vol. 3, 1995, p. 263-266.
- AAO, Focal Points: Evaluation and Surgery of the Lacrimal Drainage System in Adults, Module #12, 1995.
- AAO, Basic and Clinical Sciences Course. Section 7: Orbit, Eyelids, and Lacrimal System, 2013-2014.
- AAO, Focal Points: Evaluation of the Tearing Adult Patient, Module #8, 2002, p.3.
-
Bian Y, Scofield-Kaplan SM, Zemsky C, Campagnoli T, Ghadiali L, Dagi Glass LR, Sallee B, Belsito DV, Winn BJ. Punctal Congestion Syndrome: A Reversible, Functional Punctal Stenosis Causing Epiphora in the Setting of Chronic Pretarsal Conjunctivitis. Ophthalmic Plast Reconstr Surg. 2021 May-Jun 01;37(3S):S92-S97. doi: 10.1097/IOP.0000000000001840. PMID: 32890120; PMCID: PMC7904972.
- Chan DM, Golubev I, Shipchandler TZ, et al. Improving outcomes by combining septoplasty with primary external dacryocystorhinostomy. Am J Otolaryngol. 2014 May-Jun;35(3):309-12.
- Esmaeli B, Amin S, Valero V, Adinin R, Arbuckle R, Banay R, Do KA, Rivera E. Prospective study of incidence and severity of epiphora and canalicular stenosis in patients with metastatic breast cancer receiving docetaxel. J Clin Oncol. 2006 Aug 1;24(22):3619-22.
- Fezza JP, Wesley RE, Klippenstein KA. The treatment of punctal and canalicular stenosis in patients on systemic 5-FU. Ophthalmic Surg Lasers. 1999 Feb;30(2):105-8.
- Morgenstern KE, Vadysirisack DD, Zhang Z, et al. Expression of sodium iodide symporter in the lacrimal drainage system: implication for the mechanism underlying nasolacrimal duct obstruction in I131-treated patients. Ophthal Plast Reconstr Surg. 2005 21(5):337-344.
- Sobel RK, Carter KD, Allen RC. Bilateral lacrimal drainage obstruction and its association with secondary causes. Ophthal Plast Reconstr Surg. 2014 Mar-Apr;30(2):152-6.
- Wearne MJ, Pitts J, Frank J, et al. Comparison of dacryocystography and lacrimal scintigraphy in the diagnosis of functional nasolacrimal duct obstruction. Br J Ophthalmol 1999;83:1032-1035.
- Webber NK, Setterfield JF, Lewis FM, Neill SM. Lacrimal canalicular duct scarring in patients with lichen planus. Arch Dermatol. 2012 Feb 148(2):224-7.
