Generalized staphylococcal scalded skin syndrome
Updated May 2025
Alexis Kassotis and Lora R. Dagi Glass, MD
Establishing the diagnosis
- Staphylococcal scalded skin syndrome (SSSS) is a potentially lethal dermatologic condition.
- It is caused by strains of Staphylococcus aureus that produce exfoliative exotoxins A and B (ETA and ETB).
- These exotoxins act as serine proteases that cleave desmoglein 1 (a desmosomal protein); cleavage leads to loss of keratinocyte-keratinocyte adhesion in the stratum granulosum, which compromises skin architecture.
- Rates of SSSS due to Methicillin-Resistant S. aureus (MRSA) vary by location but are relatively low.
- Recent studies confirm an increasing recognition of MRSA-associated SSSS, especially in healthcare settings, although methicillin-sensitive S. aureus remains the predominant cause. This is important for empirical antibiotic choices and infection control (Chen 2024).
- There are two forms of SSSS:
- Localized disease (bullous impetigo), which rarely affects the periocular region.
- Generalized disease, which almost always affects the periocular region.
This review will focus on the generalized form of SSSS, which occurs when the exotoxin spreads hematogenously, causing widespread damage to the epidermis.
Epidemiology
- Estimated incidence is between 0.09 and 0.56 cases per million (Handler, 2014).
- Primarily a disease of immunocompetent children
- Disease most commonly occurs from the neonatal period (as early as 48 hours after birth) to 5 years of age.
- Antibodies against the staphylococcal exotoxins are typically acquired in childhood, which accounts for the relative rarity of disease in older children and adults.
- Infants are especially vulnerable due to immature renal function, leading to reduced clearance of exotoxins.
- Newborn renal immaturity remains a critical risk factor, but genetic polymorphisms affecting immune response are being studied as contributors to susceptibility (Yang 2021).
- Approximately one-third of healthy individuals are colonized with S. Aureus and can serve as a vector of disease (Sakr, 2018).
- Outbreaks are commonly seen in schools and day care facilities.
- Disease most commonly occurs from the neonatal period (as early as 48 hours after birth) to 5 years of age.
- SSSS is rare in adults; about 60 cases have been documented.
- Adult cases, though rare, are increasingly documented with better recognition; both immunocompetent and immunocompromised adults can be affected (Kim 2023).
- Renal impairment is a major risk factor as it leads to impaired excretion of circulating exotoxins. However, multiple cases have been reported in immunocompetent adults without renal dysfunction (Patel, 2000).
Clinical Features
- Prodrome of fever, agitation and irritability (particularly in young children), sore throat, anorexia, and conjunctivitis.
- After a non-specific prodromal period, erythroderma develops. This is followed by superficial desquamation with some areas of skin sparing. This gives the skin a wrinkled appearance and skin may slough off with gentle pressure (Nikolsky sign). After this, fragile vesicles and bullae form that then burst to reveal an erythematous base.
- Periocular crusting often occurs after lesions burst however recent case series highlight early periocular edema and erythema as potential clinical markers for prompt diagnosis, especially in neonates (Yang, 2021).
- Disease is confined to the epidermis which differentiates SSSS from Toxic epidermal necrolysis (TEN), a common mimic characterized by dermal involvement.
- Disease classically begins on the head or face (Figure 1).
- In neonates, lesions are typically also present around the umbilical stump and in the diaper area.
- Absence of mucous membrane involvement helps differentiate SSSS from similar blistering conditions including TEN and Stevens-Johnson syndrome.
- Systemic features including fever, malaise, and signs of significant dehydration such as poor skin turgor and delayed capillary refill time are usually present.
- Systemic inflammatory markers and cytokine profiles are being investigated to distinguish SSSS from mimics and predict severity (Alani 2025).
Diagnostic Tests
- Typical clinical pattern (see clinical features)
- Skin biopsy and histopathologic evaluation demonstrating:
- Detachment of the superficial epidermis beginning at the stratum granulosum.
- Lack of notable inflammation.
- Isolation of S. Aureus producing ETA or ETB
- Skin cultures with nasal swab
- Blood cultures
- Culture of the umbilical stump in neonates
Other Diagnostic Studies
- Tzanck smear
- May be combined with negative direct immunofluorescence to increase specificity.
- Gram stain (demonstrating clusters of gram-positive cocci) may be used in a resource limited setting.
- In conjunction with typical clinical features, suggestive findings on gram stain is justification to start empiric therapy.
- Testing is relatively rapid; thus, it may shorten time between presentation and treatment.
- As disease is toxin mediated, samples cannot be taken from bullae or vesicles as they will not contain bacteria.
- Dermatoscopy demonstrates erosions with peripheral epidermal remnants and can help support the diagnosis.
- Point-of-care rapid antigen tests for S. aureus exotoxins are under development and show promise for bedside diagnosis in neonates (Saleh 2025).
Differential diagnosis
- Toxic epidermal necrolysis
- Stevens-Johnson Syndrome
- Epidermolysis bullosa
- Pemphigus foliaceous
- Drug reaction with eosinophilia and systemic symptoms (DRESS)
- Staphylococcal toxic shock syndrome
Patient management: treatment and follow-up
- Medical therapy: treatment is multidisciplinary and often requires a burn unit or intensive care unit.
- Early treatment with intravenous anti-staphylococcal antibiotics (i.e. beta-lactamase resistant penicillin) is critical in decreasing morbidity and mortality.
- Flucloxacillin is a commonly used for adults and children.
- Topical mupirocin may be used to eradicate nasal colonization of S. Aureus and topical antibiotics can be added for concurrent conjunctivitis.
- Clarithromycin or cefuroxime can be used in penicillin allergy.
- Adjuvant clindamycin, vancomycin or linezolid may be used if MRSA is suspected.
- MRSA coverage is generally added empirically in areas of high MRSA prevalence or if patients fail to improve with standard therapy.
- However, compared to Staphylococcal infections overall, those associated with SSSS are less likely to be methicillin-resistant and more likely to be clindamycin-resistant.
- While beta-lactam antibiotics like cefazolin remain first-line treatments for methicillin-sensitive Staphylococcus aureus (MSSA), recent findings suggest that clindamycin, despite its known anti-toxin effects, may not significantly improve clinical outcomes in SSSS. This has led to a reevaluation of its routine use (Gray 2025).
- The combination of clindamycin with melittin (a drug with broad spectrum antibacterial, antiviral, antifungal, antiprotozoal and anti-inflammatory effects) has been recently demonstrated to have synergistic activity against Staphylococcal exfoliative exotoxin A and B (Mahmoudi, 2020).
- Fresh frozen plasma (FFP) was demonstrated in a case series to effectively neutralize antibodies against exotoxin A in the pediatric population (Tenendbaum, 2007).
- Use of intravenous immunoglobulin (IVIG) can be attempted in children who have failed other treatments, although its use may be associated with prolonged hospitalization (Li, 2013 & Handler, 2014).
- A recent case report demonstrated the efficacy of IVIG as part of a multimodal treatment regimen in an adult with SSSS (Urata, 2018).
- Analgesics (i.e. acetaminophen, fentanyl)
- Intravenous fluid resuscitation
- Nasogastric feeding
- Wound dressings
- Surgical therapy: not applicable
Natural History and Prognosis
- Bullae rupture within 24 to 48 hours and skin healing begins soon after with no scarring.
- Patient survival is excellent in children (<5% mortality) but adult mortality remains high (>60%) (Patel, 2003).
Complications
- Sepsis
- Pneumonia
- Secondary bacterial infection
- Electrolyte imbalances
- Thermal dysregulation due to skin barrier compromise
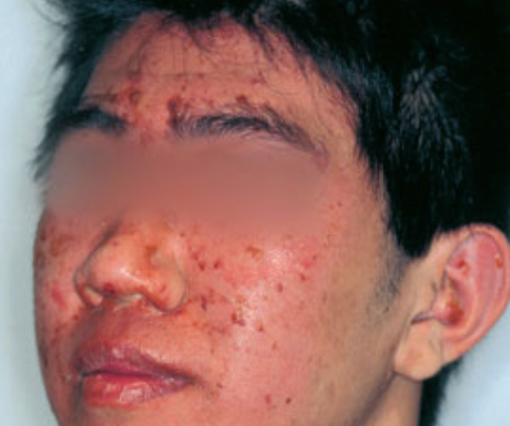
Photograph courtesy of D@nderm.
Figure 1. Early stages of staphylococcal scalded skin syndrome with diffuse facial erythema and exfoliation.
References and additional resources
- Patel GK & Finlay AY. Staphylococcal Scalded Skin Syndrome. Am J Clin Dermatol. 2003;4:165–175.
- Mockenhaupt M, Idzko M, Grosber M, Schopf E, Norgauer J. Epidemiology of staphylococcal scalded skin syndrome in Germany. J Invest Dermatol 2005; 124: 700–703.
- Patel GK, Varma S, Finlay AY. Staphylococcal scalded skin syndrome in healthy adults. Br J Dermatol. 2000;142(6):1253-1255.
- Chen W, Luo Y, Xiao Y, Deng L, Yang H, Li Y, Zhou B, He L. The Clinical Characteristics and Antimicrobial Resistance of Staphylococcus aureus Isolated from Patients with Staphylococcal Scalded Skin Syndrome (SSSS) in Southwestern China. Antibiotics (Basel). 2024;13:516.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J European Academy of Dermatol and Venereology. 2014;28(11): 1418-1423.
- Li MY, Hua Y, Wei GH, Qui L. Staphylococcal scalded skin syndrome in neonates: an 8‐year retrospective study in a single institution. Pediatr Dermatol 2013; 31: 1–5.
- Yang T, Wang J, Cao J, Zhang X, Lai Y, Li L, Ye X, You C. Antibiotic-resistant profile and the factors affecting the intravenous antibiotic treatment course of generalized Staphylococcal Scalded Skin Syndrome: a retrospective study. Ital J Pediatr. 2021;47:169.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front Microbiol. 2018;9:2419.
- Kim J, Kim GL, Norambuena J, Boyd JM, Parker D. Impact of the pentose phosphate pathway on metabolism and pathogenesis of Staphylococcus aureus. PLoS Pathog. 2023;19(7):e1011531.
- Alani O. Staphylococcal scalded skin syndrome: A rare mimicker of toxic epidermal necrolysis. Int J Dermatol. 2025;64(5):e161–e162.
- Haasnoot PJ, De Vries A. Staphylococcal scalded skin syndrome in a 4-year-old child: a case report. Journal of Medical Case Reports. 2018; 12(20).
- Levine G, Norden CW. Staphylococcal scalded skin syndrome in an adult. N Engl J Med. 1972; 287: 1339-40.
- Panwar H, Joshi D, Goel G, Asati D, Majumdar K, Kapoor N. Diagnostic Utility and Pitfalls of Tzanck Smear Cytology in Diagnosis of Various Cutaneous Lesions. J Cytol. 2017;34(4):179‐182.
- Saleh HM, Ross A, Sathe NC. Staphylococcal Scalded Skin Syndrome. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/sites/books/NBK448135/
- Morgenstern-Kaplan D, Fonseca-Portilla R, Konstat-Korzenny E, Cohen-Welch A. The Role of Gram Staining in Staphylococcal Scalded Skin Syndrome. Cureus. 2020;12(4):e7624.
- Gil Sáenz FJ, Herranz Aguirre M, Durán Urdániz G, et al. Clindamycin as adjuvant therapy in staphylococcal skin scalded syndrome. An Sist Sanit Navar. 2014;37(3):449–53.
- Gray L. Pediatric Staphylococcal Scalded Skin Syndrome: A Review of Current Treatment Approaches. Pediatr Dermatol. 2025;42(4):e123–e126.
- Mahmoudi H, Alikhani MY, Imani Fooladi AA. Synergistic antimicrobial activity of melittin with clindamycin on the expression of encoding exfoliative toxin in Staphylococcus aureus. Toxicon. 2020;183:11‐19.
- Wang Z, Feig JL, Mannschreck DB, Cohen BA. Antibiotic sensitivity and clinical outcomes in staphylococcal scalded skin syndrome. Pediatr Dermatol. 2020;37(1):222‐223.
- Tenendbaum T, Hoehn T, Hadzik B et al. Exchange transfusion in a preterm infant with hyperbilirubinemia, staphylococcal scalded skin syndrome (SSSS) and sepsis. Eur J Pediatr 2007; 166: 733–735.
- Li MY, Hua Y, Wei GH, Qiu L. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31(1):43-47.
- Urata T, Kono M, Ishihara Y, Akiyama M. Adult Staphylococcal Scalded Skin Syndrome Successfully Treated with Multimodal Therapy Including Intravenous Immunoglobulin. Acta Derm Venereol. 2018;98(1):136-137.
- Blyth M, Estela C, Young AE. Severe staphylococcal scalded skin syndrome in children. Burns. 2008; 34: 98–103.
Financial disclosures
Reviewers
Sana Ali: No disclosures
