Genetics for the Oculofacial and Orbital Surgeon
Updated May 2024
Luke (Jin Kyun) Oh and Megan Soucy, MS, LCGC
What is genetic testing and why do we care?
- Genetic testing is used to analyze the DNA of an individual for variants that may lead to clinically relevant disease.
- Next-generation sequencing
- DNA copied hundreds of times in tiny fragments which are concurrently sequenced and mapped back against a reference genome.
- Allows for the cost of genetic testing to continue to decline substantially.
- Precision medicine
- Novel approach to patient care that takes into account the impact of an individual’s genes, environment, and lifestyle on health and disease.
- Currently applications include cancer therapeutics and risk scores, pharmacogenomics, fetal gene therapy, and ocular gene therapy.
- Upcoming therapeutic applications
- Over 4,500 ongoing or completed gene therapy trials for currently untreatable diseases, roughly 100 of which are for ophthalmic disease.
- Numerous disease-causing genes and associations have yet to be discovered.
- Genetic disorders are responsible for a significant proportion of healthcare expenditure.
- Estimated to account for 40-50% of costs in the pediatric population (Miller et al. 2020, Front Public Health).
What are the types of genetic testing available?
- Karyotype/Chromosome Analysis
- Examination of the structure and number of chromosomes to identify abnormalities such as deletions, duplications and translocations.
- Only technology that can identify balance translocations.
- Applications: Used to diagnose aneuploidy, Down Syndrome, find causes of infertility, or diagnose cancers such as chronic myeloid leukemia.
- Relative Cost: $500-$700
- Time to results: 2 weeks
- Insurance Coverage: Typically covered
- Examination of the structure and number of chromosomes to identify abnormalities such as deletions, duplications and translocations.
- Chromosome Microarray
- Detects small differences in the chromosome content between a patient’s DNA and control DNA.
- May utilize single nucleotide polymorphism technology which can also detect areas of homozygosity
- Applications: Significantly more sensitive in identifying microdeletions and duplications than traditional karyotype.
- Frequently used in patients with developmental abnormalities that are not specific for a clear genetic disorder.
- Relative Cost: $1500-$2000
- Time to results: 2-3 weeks
- Insurance Coverage: Typically covered for syndromic conditions and fetal testing.
- Detects small differences in the chromosome content between a patient’s DNA and control DNA.
- Targeted Sequencing
- Sequencing of a specific variant, gene or region of interest to diagnose a cause of hereditary disease.
- Applications: Used in the setting of a high degree of suspicion for a specific disease entity.
- Relative Cost: $100-$500
- Time to results: Within 4 weeks
- Insurance Coverage: Typically covered for symptomatic individuals, but not for family member testing.
- Panel testing
- Sequencing multiple genes simultaneously due to genetic heterogeneity or an unclear clinical diagnosis.
- Allows for great read depth which increases sensitivity. Captures insertion/deletion analysis, copy number analysis, known intronic mutations and difficult to sequence regions such as open reading frames and trinucleotide repeats.
- Applications: Used when targeted sequencing of each potentially causative gene is impractical or if there are several conditions on a differential and one wants to test them simultaneously for a quicker result.
- Relative Cost: $250-$4,000
- Time to results: 8-12 weeks
- Insurance Coverage: Typically covered if phenotype of the patient matches the tested panel.
- Sequencing multiple genes simultaneously due to genetic heterogeneity or an unclear clinical diagnosis.
- Whole exome sequencing
- Sequencing of the entire protein-encoding portion of the genome (~1-2%).
- Cannot detect insertion/deletion analysis, copy number variants, intronic regions, and many difficult to sequence regions of DNA.
- Application: Designed for syndromic conditions but increasingly used as a more cost effective method of testing for non-syndromic conditions.
- Roughly 85% of identified disease-causing mutations are located within exons (Rabbani et al. 2014, J Hum Genet).
- Diagnostic yield of between 20-60%, increased by utilizing a trio for analysis by 16% (Stark et al. 2016, Farwell et al. 2015, Genet Med).
- Relative Cost: $2,500 – $6,000
- Time to results: 8 weeks – 6 months
- Insurance Coverage: Often not covered for non-syndromic conditions.
- Sequencing of the entire protein-encoding portion of the genome (~1-2%).
- Whole genome sequencing
- Sequencing of the entire human genome including the non-coding portions.
- Application: Most comprehensive genetic test available.
- Diagnostic yield may increase by roughly 30% compared to more targeted testing (Ellingford et al. 2016, Ophthalmology).
- Often utilized in the setting of previously unsuccessful diagnostic testing.
- Relative Cost:$3500-$16,000
- Time to results: 8 weeks to several months
- Insurance Coverage: Covered by select private and Medicaid insurances with strict eligibility requirements.
Who should receive genetic testing and what is the role of genetic counseling?
- Considerations prior to genetic testing
- Who is best testable?
- The best person to start genetic testing in the family is the person who is affected and has the most suspicion for genetic disease based on pathology, age of onset, and/or bilateral disease
- What are you trying to achieve?
- Clinical testing should be done to confirm or establish a diagnosis, help with family planning, or guide treatment.
- Research sequencing should be done in order to discover new genetic causes for disease.
- Who is best testable?
- Ethical concerns & legal
- Non-paternity is often discovered by genetic testing due to the necessity of having to test reported parents to determine genetic arrangement.
- Traditionally reported rate of 10% globally.
- Studies suggest rates range from 0.8-30% (median 3.7%) and that the higher the paternal confidence, the lower the discordance (Bellis et al. 2005 J Epidemiol Community Health)
- Genetic Information Nondiscrimination Act of 2008 provides protections against discrimination based on genetic information for employment and health insurance.
- It has limited protections for active military personnel and does not cover long term care, disability, and life insurance.
- Privacy concerns about what is being done with the leftover DNA and the data generated from the sequence as well as who has access to it.
- Testing minors is an issue when it comes to predictive testing as it removes the minor’s right not to know. It may cause psychological harm to the patient, as many conditions do not have risk-reducing interventions or treatment. Others argue that it may change how parents wish to raise their children and how children will plan their future.
- The legacy of eugenics and harm from medical research has made certain populations skeptical of participating in genetic testing for their health.
- Non-paternity is often discovered by genetic testing due to the necessity of having to test reported parents to determine genetic arrangement.
- Family screening
- Cascade testing can occur once genetic causes are identified in a family, starting with first degree relatives.
- Genetic counseling
- The act of actively engaging patients in a partnership to make decisions based on their genetic health.
- Personalized risk assessment for a genetic condition based on family and personal history.
- Discussions about risks, limitations, and benefits of genetic assays.
- Education about the genetic conditions, inheritance patterns and genetic testing.
- Review of medical management recommendations and lifestyle changes that can be helpful.
- Reproductive risks and familial risks with identification of individuals who should follow up with genetic testing.
- The act of actively engaging patients in a partnership to make decisions based on their genetic health.
- Genetics professionals who can help
- Genetic counselors
- Genetic professionals who have specialized training in genetics and counseling to assist patients with decisions about their genetic health and/or better understand their genetic testing results (https://www.aboutgeneticcounselors.org/Who-Are-Genetic-Counselors/Who-Are-Genetic-Counselors).
- Many genetic counselors work in hospital or clinic settings with doctors to assist with ordering and interpreting genetic testing and relaying information that is digestible to patients.
- To find a genetic counselor near you go to: https://findageneticcounselor.nsgc.org.
- Genetic nurses
- Licensed professional nurses with special education and training in genetics (https://www.isong.org/page-1325153).
- Can provide genetic counseling to patients and provide nursing care for patients and family members with or at risk of hereditary disease.
- Medical geneticist
- Clinical geneticists are physicians who are American Board of Medical Genetics and Genomics (ABMGG) certified (https://www.acmg.net/ACMG/Education/Student/Careers_in_Medical_Genetics.aspx).
- Specialize in clinical evaluation, diagnosing, management and treatment of genetic conditions across all ages. Can perform dysmorphology examination and give a clinical diagnosis, if one exists, without having to do genetic testing. Evaluation and sign off may be necessary for some genetic testing
- To find a genetics clinic: https://clinics.acmg.net/
- Genetic counselors
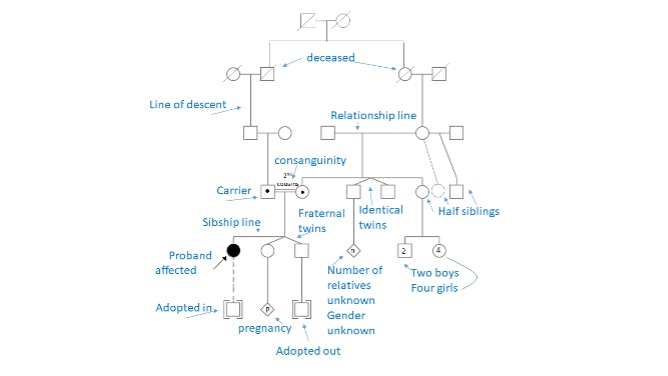
- How to interpret a pedigree
- Labeled pedigree below. Full standardized pedigree nomenclature available at Bennet et al. 2008, J Gent Couns
Analysis and interpretation of results
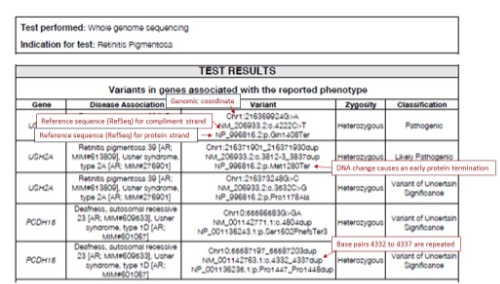
- Variants identified on DNA testing will be noted using HGVS nomenclature. Full nomenclature rules can be found at http://varnomen.hgvs.org/.
- The coding DNA reference sequence is denoted as “c.”
- The protein reference sequence is denoted as “p.”
- Intronic variants located at the beginning of an intron are noted with the last basepair of the exon a “+” and then the number of base pairs until until the mutation
- Intronic variants located at the end of an intron are noted with the first base pair of the following exon and then a “-” and then the number of base pairs until the mutation
- Substitutions are typically shown as old base pair > new base pair
- Deletions may be noted del; Duplications may be noted as dup
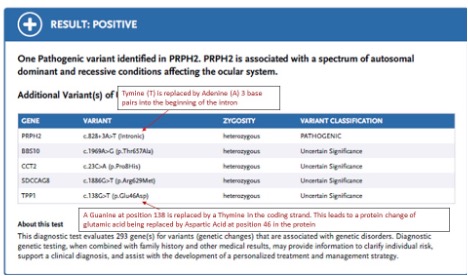
- Variants identified through sequencing are classified based according to a five-tier system as recommended by the American College of Medical Genetics.
- Pathogenic: The variant is known to cause disease with ~99% certainty.
- Likely Pathogenic (LP): There is greater than ~90% certainty that the variant contributes to disease.
- Variant of Uncertain Significance (VOUS): There is not enough information to determine if the variant does or does not cause disease.
- Likely Benign: There is greater than ~90% certainty that the variant does not cause disease.
- Benign: The variant is known not to cause disease with ~99% certainty.
- Variants may be reclassified based upon additional evidence
- It is the duty of the provider to inform the patient of the reclassification as it may have a significant impact on patient management or screening.
- Phase testing determines which alleles are inherited from the maternal and paternal chromosomes
- Used to confirm if variants are on one allele or separate alleles in recessive conditions.
- Frequently a requirement for gene therapy involving recessive conditions.
- Clinical correlation of genetic testing results is essential, as mutations may have reduced or absent penetration and diseases often present with variable phenotypic expression.
- Negative results on genetic testing can have two different implications
- A true negative test suggests the patient is negative for the identifiable genetic cause of disease in the family.
- An uninformative negative test suggests that the patient may have a true increased risk of disease, but no causative mutation was identified, or familial risk cannot be eliminated.
Practical and potential applications of genetic testing in oculoplastics
- Gene therapy becomes available for oculoplastic disease
- Gene therapy involves the addition, removal, or correction of DNA in an individual’s genome to manipulate defective genes that may cause disease.
- Traditionally uses adeno-associated viruses or lentivirus vectors for gene insertion.
- CRISPR-Cas9 gene editing technology has revolutionized gene therapy and allows for targeted correction of specific gene alterations.
- CRISPR-Cas9 is currently being utilized in several clinical trials.
- Several disease entities that oculoplastic surgeons may encounter are being studied as candidates for gene therapy.
- Lamellar ichthyosis (TGM1)is in Phase I/II trial for a topical gene therapy (NCT04047732).
- Requires genetic testing for TGM1 in order to be enrolled.
- Basal cell nevus syndrome (PTCH1, PTCH2, SUFU) is currently in Phase II trial for injectable gene therapy targeting IFNG (NCT04416516).
- Melanoma is studied in several gene therapy trials seeking to augment immune modulators such as GMSF, TCR, or IL-2.
- Lamellar ichthyosis (TGM1)is in Phase I/II trial for a topical gene therapy (NCT04047732).
- Gene therapy for disorders such as chronic progessive external ophthalmoplegia (CPEO) (Flierl et al. 2005, Gene Ther) and optic gliomas and schwannomas in neurofibromatosis type 1 and 2 have been successfully treated in preclinical animal models (Freret et al. 2019, J Neurosci Res, Prabhakar et al. 2013, Hum Gene Ther).
- Gene therapy involves the addition, removal, or correction of DNA in an individual’s genome to manipulate defective genes that may cause disease.
- Discovery of novel genes or genetic associations that cause oculoplastic disease
- Nearly half of diseases with an underlying genetic etiology have no identified causative gene.
- Numerous oculoplastic disorders are known to have a genetic component in disease pathogenesis, such as thyroid eye disease.
- Gene discovery is an important step to improve understanding of disease pathophysiology and guide therapeutic endeavors.
- Congenital ptosis has a number of candidate genes (ZFH4, COL25A1) but no known causative gene (McMullan et al.2002, Hum Genet., Khan et al. 2015, J AAPOS).
- Several conditions such as congenital ectropion, congenital alacrimia, blepharo-cheilo-odontic syndrome have no known etiology.
- Nearly half of diseases with an underlying genetic etiology have no identified causative gene.
- Risk management and family planning for patients with genetic disease and oculoplastic manifestation
- Genetic disorders are frequently chronic and require long term care that may influence patient prognosis.
- Early identification of symptoms and diagnosis may improve outcomes.
- Ectrodactyly-ectodermal dysplasia-cleft (EEC) syndrome requires long term management of the anterior segment to prevent keratopathy and lacrimal dysgenesis (Elmann et al. 2015, Ophthalmic Plast Reconstr Surg, Kennedy et al. 2015, Cont Lens Anterior Eye)
- Diagnosis of genetic disorder may affect family planning and testing in the spouse, children, and family members.
- Conditions such as EEC or neurofibromatosis type 1 and 2 have phenotypic variability and require genetic counseling to discuss risk and familial diagnosis (Radtke et al. 2007, J Genet Couns).
- Early identification of symptoms and diagnosis may improve outcomes.
- Genetic disorders are frequently chronic and require long term care that may influence patient prognosis.
- Different genetic etiologies may influence medical management of the patient
- Congenital myasthenic syndromes can be caused by mutations in numerous genes (CHAT, CHRNE, COLQ, DOK7).
- Different therapeutic interventions are indicated based upon the gene implicated in the disease (Lorenzoni et al. 2012, Pediatr Neurol).
- Congenital myasthenic syndromes can be caused by mutations in numerous genes (CHAT, CHRNE, COLQ, DOK7).
References and additional resources
- Miller KE, Hoyt R, Rust S, et al. The Financial Impact of Genetic Diseases in a Pediatric Accountable Care Organization. Front Public Health. 2020;8:58.
- Rabbani B, Tekin M, Mahdieh N. The promise of whole-exome sequencing in medical genetics. J Hum Genet. 2014;59:5-15.
- Stark Z, Tan TY, Chong B, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18:1090-1096.
- Farwell KD, Shahmirzadi L, El-Khechen D, et al. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet Med. 2015;17:578-586.
- Ellingford JM, Barton S, Bhaskar S, et al. Whole Genome Sequencing Increases Molecular Diagnostic Yield Compared with Current Diagnostic Testing for Inherited Retinal Disease. Ophthalmology. 2016;123:1143-1150.
- Bellis MA, Hughes K, Hughes S, Ashton JR. Measuring paternal discrepancy and its public health consequences. J Epidemiol Community Health. 2005;59:749-754.
- Who Are Genetic Counselors? Available at: https://www.aboutgeneticcounselors.org/Who-Are-Genetic-Counselors/Who-Are-Genetic-Counselors. Accessed March 7th, 2021.
- Find a Genetic Counselor. Available at: https://findageneticcounselor.nsgc.org/. Accessed March 7th, 2021.
- International Society of Nurses in Genetics. Available at: https://www.isong.org/page-1325153. Accessed March 7th, 2021.
- Careers in Medical Genetics. Available at: https://www.acmg.net/ACMG/Education/Student/Careers_in_Medical_Genetics.aspx. Accessed March 7th, 2021.
- Find a Genetic Clinic. Available at: https://clinics.acmg.net/. Accessed March 7th, 2021.
- Bennett RL, French KS, Resta RG, Doyle DL. Standardized human pedigree nomenclature: update and assessment of the recommendations of the National Society of Genetic Counselors. J Genet Couns. 2008;17:424-433.
- Sequence Variant Nomenclature. Available at: http://varnomen.hgvs.org. Accessed March 7th, 2021.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424.
- Topical KB105 Gene Therapy for the Treatment of TGM1-deficient Autosomal Recessive Congenital Ichthyosis (ARCI). Available at: https://clinicaltrials.gov/ct2/show/NCT04047732. Accessed March 7th, 2021.
- Safety and Efficacy of ASN-002 Combined With a Hedgehog Pathway Inhibitor. Available at: https://clinicaltrials.gov/ct2/show/NCT04416516. Accessed March 7th, 2021.
- Flierl A, Chen Y, Coskun PE, et al. Adeno-associated virus-mediated gene transfer of the heart/muscle adenine nucleotide translocator (ANT) in mouse. Gene Ther. 2005;12:570-578.
- Prabhakar S, Taherian M, Gianni D, et al. Regression of schwannomas induced by adeno-associated virus-mediated delivery of caspase-1. Hum Gene Ther. 2013;24:152-162.
- McMullan TW, Crolla JA, Gregory SG, et al. A candidate gene for congenital bilateral isolated ptosis identified by molecular analysis of a de novo balanced translocation. Hum Genet. 2002;110:244-250.
- Khan AO, Al-Mesfer S. Recessive COL25A1 mutations cause isolated congenital ptosis or exotropic Duane syndrome with synergistic divergence. J AAPOS. 2015;19:463-465.
- Elmann S, Hanson SA, Bunce CN, Shinder R. Ectrodactyly ectodermal dysplasia clefting (EEC) syndrome: a rare cause of congenital lacrimal anomalies. Ophthalmic Plast Reconstr Surg. 2015;31:e35-7.
- Kennedy DP, Chandler JW, McCulley JP. Ocular surface involvements in ectrodactyly-ectodermal dysplasia-cleft syndrome. Cont Lens Anterior Eye. 2015;38:228-231.
- Radtke HB, Sebold CD, Allison C, et al. Neurofibromatosis type 1 in genetic counseling practice: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2007;16:387-407.
- Lorenzoni PJ, Scola RH, Kay CS, Werneck LC. Congenital myasthenic syndrome: a brief review. Pediatr Neurol. 2012;46:141-148.
