Conjunctival Epithelial Malignancies
Updated May 2025
Navdeep Nijhawan, MD; Ahsen Hussain, MD
Ocular surface squamous neoplasia (OSSN) encompasses a spectrum of precancerous and cancerous epithelial abnormalities of the conjunctiva and cornea. This spectrum includes benign lesions such as papilloma, pseudoepitheliomatous hyperplasia and benign hereditary intraepithelial dyskeratosis. It also includes preinvasive lesions like conjunctival intraepithelial neoplasia (CIN) and invasive lesions such as squamous cell carcinoma (SCC) and mucoepidermoid carcinoma (MEC). (Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea 2005).
Establishing the diagnosis
Etiology
Incidence of OSSN is approximately 0.03 to 1.9 per 100,000 persons in the United States and Australia. In African populations, the incidence of OSSN has been estimated between 1.6 and 3.4 per 100,000. The higher rates compared to the United States and Australia likely attributed to HIV-related immunosuppression (Greenfield JA et al, Cornea, 2024).
The conjunctival epithelium consists of both stratified squamous and columnar epithelium. Squamous epithelium is found near the limbus changing to the columnar type near the fornix.
Actinic induced dysplasia is considered the predominant etiology with the conjunctiva being the only mucous membrane in relatively constant exposure to sunlight.
Epidemiology
In temperate climates, the highest prevalence is in Caucasian males over the age of 60, range 20–88 years (Lee GA, Arch Ophthalmol 1992; Shields et al., Ophthalmology Sept 2004).
There is generally a male over female preponderance, however prevalence affects genders equally in Africa (Gichuhi et al. Exp Eye R, 2014).
The average age of incidence of carcinoma in situ lesions is 5–9 years lower than invasive OSSN, this difference representing the time taken for progression from CIN to invasive carcinoma.
It is recognized that those populations living within 30 degrees latitude from equator have increased incidence. (Newton et al. The Lancet 1996).
Also, studies have shown an increased rate associated with HIV infection. (Osahon et al. Asian Pac J Trop Biomed, 2011).
Recurrence and mortality rates are not clearly established due to a paucity of prospective studies and differing follow-up periods.
Significant predictors for recurrence are size of lesion, positive surgical margins, elevated proliferation index measured by Ki-67 score, and increased age (McKelvie P, BJO 2002).
Recurrence rates can reach up to one-third of patients; variations due to treatment and follow-up differences across centers (McKelvie P, BJO 2002).
Recurrences typically occurs within 2 years after removal.
Mortality ranges from 0% to 8% across various studies. Metastasis is not necessarily a predictor of death in OSSN (McKelvie P, BJO 2002).
History
The patient may complain of pain, irritation, and hyperemia, occasionally of a pre-existing pterygium site. Lee GA et al., Aust NZ J Ophthalmol 1997 determined ocular irritation to predict a 2.4-fold increase in recurrence (after treatment) in patients with OSSN. Patients may also be asymptomatic.
Inquire regarding a history of increased sun exposure, hereditary, and acquired immunodeficient conditions (including HIV infection) and HPV infection.
The diffuse type of OSSN is rare and may present early as persistent conjunctival redness without follicles or papillae. Involvement of the palpebral conjunctiva can cause lid margin inversion, making it resemble chronic conjunctivitis (Basti et al, Cornea, 2003).
Clinical features
Most commonly occur on the bulbar conjunctiva in the limbal region on the nasal side (Wadell et al. 2006), and typically arises over a pre-existing area of solar elastosis such as a pterygium.
OSSN is characterized by epithelial thickening with possible extension onto the cornea. Corneal involvement may only appear as a subtle, gray surface opacity sometimes only discernible on slit-lamp biomicroscopy (hence the need for vigilant examination before surgery).
CIN typically presents as a fleshy, sessile or slightly elevated lesion, typically at the limbus within the interpalpebral fissure, and less often in the forniceal or palpebral conjunctiva. It may extend onto the adjacent corneal epithelium, and surface leukoplakia can occur due to hyperkeratosis (Shields et al, Indian J Ophthalmol, 2019).
Clinically, symptoms may range from asymptomatic to a chronically irritated, red eye. Masses are initially mobile; the conjunctiva in later stages becoming fixed to the globe with deeper scleral infiltration.
OSSN and MEC are difficult to distinguish clinically; key findings of these placoid, gelatinous or fern-like lesions are the coexistence of a skein of corkscrew or hairpin vessels indicating an underlying sessile papillary architecture. A feeder vessel may be seen.
The involved epithelium may also be subtly opalescent and show punctate staining with supravital dyes (Rankin et al., Surv Ophth July-Aug 2012).
Surface keratinization is not pathognomonic for OSSN and may be seen over any elevated lesion not covered by an adequate tear film; however it should arouse suspicion particularly when correlated to a leukoplakic lesion.
MEC is a rare variant of OSSN occurring in older patients with a yellow-globular cystic component due to the presence of multiple mucus secreting cells within cysts. This lesion (as with the spindle cell variant of SCC) is more aggressive and requires detailed clearance as well as closer follow-up (Shields CL, Shields JA, Oculoplastics and Orbit, Springer 2006).
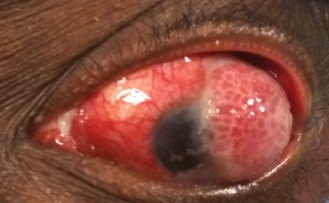
Figure 1. Ocular surface squamous neoplasia.
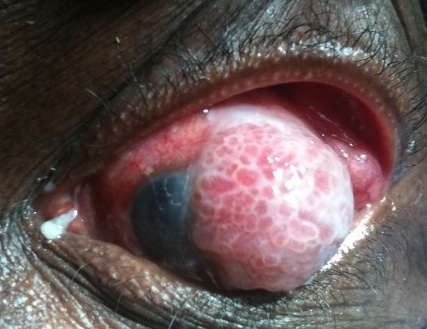
Figure 2. Advanced ocular surface squamous neoplasia of the conjunctiva.
Testing
An incisional or excisional biopsy is usually recommended to confirm the diagnosis; the latter technique is preferably for relatively smaller lesions (involving 4 clock hours or less of the limbus or 15mm or less in basal dimension).
Occasionally exfoliative cytology and fine-needle aspiration biopsy can provide useful information on the basis of a few cells (Spinak et al. Surv Ophthalmol 1977).
Cytologic evaluation of OSSN is useful for diagnosing corneal and conjunctival tumors, monitoring tumor response during medical treatment, and detecting recurrences during post-treatment follow-up. However, it is not possible to predict invasion based on cytology alone (Basti et al. Cornea 2003).
Clinical photography, high-resolution OCT, and in-vivo confocal laser scanning microscopy (CLSM) have been reported as recent methods to aid diagnosis, determine treatment effects and monitor for recurrence (Xu Y et al., Eye (Lond) 2012; Shousha MA Ophthalmology 2011; Parrozzani R Eye (Lond) 2011).
High-resolution OCT may show thickened hyper-reflective epithelium with abrupt transition from normal to hyper-reflective epithelium (Shousha MA, Ophthalmology 2011).
CLSM may reveal features such as
- Large areas of superficial cell/keratin debris accompanied by syncytial-like groupings
- Loss of the normal structure of the conjunctival epithelium and/or of the corneal basal epithelial layer
- Papillomatous organization
- Large fibrovascular structures
- Fine vessels perpendicular to the tumor surface
- Cellular anisocytosis, pleocytosis and anisonucleosis (Parrozani R et al., Eye (Lond) 2011)
- Enlarged nuclei with a high nuclear to cytoplasmic ratio, high reflective cytoplasm and indistinct cytoplasmic borders (100% of 10 cases described by Parrozani et al)
Interruptions of the layered epithelial structure along with cellular changes such as enlargement and polymorphic nuclei may be more predictive of transition to CIN or frank SCC (Falke K, Klin Monbl Augen Jul 2012).
Histopathological diagnosis remains the key tool in diagnosis:
- The epithelium exhibits hyperplasia, loss of goblet cells, loss of normal cell polarity, nuclear hyperchromasia and pleomorphism as well as mitotic figures.
- Dyskeratosis may be seen.
- A chronic inflammatory process may be present within the substantia propria.
- Degree of epithelial layer change and integrity of the basement membrane determines classification as intraepithelial (CIN), carcinoma in situ, or frank invasive carcinoma (SCC).
- Cellular atypia determines CIN grading into mild (basal one-third), moderate (inner two-thirds) or severe (full thickness)- this has not been correlated with prognosis.
- Severe atypia results in full-thickness epithelium involvement often with squamous eddies or keratin pearls (carcinoma in situ).
- Invasion of the stroma by neoplastic cells is required for a diagnosis of invasive carcinoma/SCC.
- Immunostaining with antibody to Ki-67, which is a nuclear antigen expressed in proliferating cells, allows evaluation of the growth fraction of normal and neoplastic cells allowing prognostic information (McKelvie P, BJO 2002).
- In OSSN, p53 mutations have been consistently detected in 48% – 55% of cases with mild to moderate conjunctival dysplasia and conjunctival intraepithelial neoplasia (CIN), and in 52% – 80% of cases with conjunctival squamous cell carcinoma (SCC) (Kounatidou, Ocul Surf, 2025).
Testing for staging, fundamental impairment
Grading based on description of OSSN by Lee and Hirst (Lee GA, Hirst LW Surv Ophthalmol 1995):
- I. Benign dysplasia
- Papilloma
- Pseudotheliomatous hyperplasia
- Benign hereditary intraepithelial dyskeratosis
- II. Preinvasive OSSN
- Conjunctival/corneal carcinoma in situ (formerly known as Bowen’s disease, conjunctival squamous dysplasia, intraepithelial epithelioma, epithelial dyskeratosis or carcinoma in situ (CIS))
- III. Invasive OSSN
- Squamous carcinoma
- Mucoepidermoid carcinoma
Staging based on the AJCC (American Joint Committee on Cancer) (Edge SB et al., AJCC Cancer Staging Manual 2009)
- TX – Primary tumor cannot be assessed
- T0 – No evidence of primary tumor
- Tis – Carcinoma in situ
- T1 – Tumor (≤5 mm in the greatest dimension) invades through the conjunctival basement membrane without invasion of adjacent structures
- T2 – Tumor (>5 mm in greatest dimension) invades through the conjunctival basement membrane without invasion of adjacent structures
- T3 – Tumor invades adjacent structures (excluding the orbit)
- T4 – Tumor invades the orbit with or without further extension
- T4a – Tumor invades orbital soft tissue without bone invasion
- T4b – Tumor invades the bone
- T4c – Tumor invades adjacent paranasal sinuses
- T4d – Tumor invades the brain
Risk factors
At the heart of malignant transformation is nonlethal DNA alteration either through mutation or damage. Key risk factors in the etiology of OSSN and MEC are
- Solar ultraviolet radiation with UVA causing indirect DNA damage via reactive oxygen species and UVB causing direct damage by crosslinking adjacent bases. Relatively constant exposure of this mucus membrane to sunlight places it at significant risk, particularly the nasal conjunctiva due to light focusing (Gichuhi S et al. Exp Eye Res 2014)
- Human papilloma virus infection particularly subtypes 6,11,16 and 18 (Pe’er J, Ophthalmol Clin North Am 2005)
- Immunodeficient states leading to failure of DNA repair mechanisms, tumor surveillance and tumor suppression, in particular noted systemic malignancy and inherited conditions such as xeroderma pigmentosum. It is estimated that the risk of conjunctival malignancies increases 13-fold in patients with HIV (Margo CE, Arch Ophthalmol 1996).
- Vitamin A deficiency compromising ocular surface integrity (allowing HPV entry), leading to cell-mediated immunodeficiency and dysregulation of stem cell differentiation
- Chronic inflammatory and cicatricial processes such as OMMP, chronic blepharoconjunctivitis, atopic eczema
- Benign processes such as pinguecula and pterygium as these are important differential diagnoses that can be confused
- Although there is no significant evidence, other factors such as cigarette smoking, petroleum products, European ancestry, chronic contact lens use and topical cyclosporine A have been proposed as possible factors (Alves LF, Arq Bras Oftalmol 2011).
Differential diagnosis
(Gupta S et al., Delhi J Ophth 2012)
- Pannus
- Actinic disease
- Vitamin A deficiency
- Benign intraepithelial dyskeratosis
- Pinguecula
- Pterygium
- Pyogenic granuloma
- Keratoacanthoma
- Pseudoepitheliomatous hyperplasia
- Malignant melanoma and nevi (in particular the amelanotic form)
- Pseudopterygia (eg, chemical or thermal burn, trauma, marginal corneal disease)
Patient management: treatment and follow-up
Natural history
Untreated, OSSN is likely to progress through its stages into frank invasive carcinoma with its inherent high mortality rate.
Depending on presumed diagnosis as well as on patient factors and the size and location of the lesion, management can include
- Serial observation (usually reserved for benign, asymptomatic lesions)
- Incisional (avoid for melanocytic tumors) or excisional biopsy
- Cryotherapy
- Chemotherapy
- Radiotherapy
- Photodynamic therapy (PDT)
- Modified enucleation
- Orbital exenteration
- Various combinations of these methods (Shields CL, Shields JA, Oculoplastics and Orbit, Springer 2006)
Medical therapy
The use of topical chemotherapeutic agents has the advantage of treating the whole ocular surface without positive margins. They are often used in conjunction with surgery to improve tumor clearance and reduce recurrence rates as dysplastic cells may extend beyond visible margins. Some authors have recently advocated multi-chemotherapy without surgery as a possible treatment strategy (Stone DU, Cornea 2005). Chemotherapy is also becoming an increasingly attractive option for management of recurrences rather than surgery (Tsatsos M, Exp Rev Ophthal 2013).
Currently used agents in the chemotherapeutic management of OSSN include (Nanji AA, Curr Opin Ophthalmol 2013):
- Mitomycin-C (MMC): An antimetabolite that alkylates DNA and disrupts the production of RNA, a traditional topical agent particularly successful for SCC especially after tumour recurrence. Resolution rates are between 82%–100% across studies (Nanji AA, Curr Opin Ophthalmol 2013). Side-effects have somewhat limited use for OSSN with studies detailing short-term complications in up to 60% of patients and long-term complications in up to 17% of patients (Nanji AA, Curr Opin Ophthalmol 2013).
- Hirst (2007) conducted a placebo controlled trial in 48 patients with ocular surface squamous neoplasia treating 26 with mitomycin-C at a dose of 0.4 mg/ml, one drop four times a day for three weeks, and 20 with placebo.
- Successful treatment was complete resolution of the neoplasia on slit lamp exam
- None of the 20 placebo patients had resolution, 24 of 26 mitomycin treated resolved.
- Patients complained of redness and irritation at 2-3 weeks but none discontinued treatment
- Response was first seen at three weeks.
- No additional topical treatment was needed for symptomatic relief.
- 5-fluorouracil (5-FU): Blocks DNA synthesis by acting as a pyrimidine analog after incorporation into RNA and is the earliest chemotherapy agent used (Keizer et al. 1986) with a good safety profile for management of low-grade CIN to SCC. In the largest series of single therapy to date, an average of 1.9 cycles of treatment was required with complete resolution in 100% of cases. Recurrence rates range from 7-20% (Nanji AA, Curr Opin Ophthalmol 2013). No long-term complications have yet been described relating to this treatment indication.
- Interferon a2b (IFN-a2b): A cytokine produced by immune cells to combat microbes and viruses and is a newer agent with improved tolerance and safety profile. Shah et al., Arch Ophthalmol 2012 demonstrated complete OSSN resolution according to the AJCC classification, in 67% of Tis cases, 85% of T3 and 83% overall. 8 of 20 patients experienced adverse effects only during treatment, resolving within 1 month of medication discontinuation. Kim et al., Ophthalmology 2012 managed giant OSSN with IFN-a2b and attained 72% complete control and 28% immunoreduction of size.
- Anti-VEGF agents: Anti-vascular endothelial growth factor agents are newly shown to be effetive in both size and vascularity of the OSSN after 3 months treatment with bevacizumab with little effect on cornea. (Faramarzi A, Cornea 2013) (Özcan AA, et al, Cornea 2014).
- Retinoic acid: Krilis et al., Ophthalmology 2012 demonstrated a possible synergistic effect of retinoic acid and IFN-a2b with 97.75% of their 89 eyes showing complete clinical resolution. No recurrence was noted at a range of follow-up of 11-84 months (mean 51.5 months).
- PDT with verteporfin has been used to show tumor regression in OSSN localized to the conjunctiva (Barbazetto IA, Am J Ophth 2004).
- Programmed death-1 (PD-1): Anti-PD-1 therapy has emerged as a promising treatment option for advanced OSSN, particularly in cases resistant to conventional therapies. Immune checkpoint inhibitors, such as pembrolizumab and cemiplimab, target the PD-1/PD-L1 pathway, which is associated with T-cell suppression and tumor immune evasion. Case studies have shown systemic administration of these agents can lead to complete or stable tumor regression in patients with advanced conjunctival squamous cell carcinoma, especially those who declined orbital exenteration. However, high treatment costs in the U.S. remain a significant barrier, although assistance programs and insurance coverage may help mitigate the financial burden. (Monroy D, et al, Eye (Lond), 2023)
Radiation
External beam radiotherapy (EBRT) and custom-designed plaque radiotherapy can be used in the management of primary or residual/recurrent OSSN.
Proton and electron EBRT are favored in OSSN as they limit radiation exposure to the tumor and minimize negative effects on the normal surrounding tissue.
EBRT to a total dose of 3,000–4,000 cGy is usually reserved for large tumors with a poor prognostic factors and patient factors precluding other modes of treatment.
Plaque radiotherapy has had a recent resurgence as an adjunctive and salvage treatment for OSSN.
- Arepalli et al., JAMA Ophthalmol 2014 recently demonstrated plaque radiotherapy as an alternative to enucleation in patients with residual SCC after surgical excision.
- Lecuona et al., BJO 2015 demonstrated complete resolution with no recurrences of SCC or CIN in patients with HIV treated with a larger 18-mm Sr-90 brachytherapy plaque after surgical clearance with a 2-mm margin.
Surgery
Surgery with a no-touch technique and at least a 3 to 4 mm uninvolved margin remains a mainstay of treatment whether for only initial tissue diagnosis or formal excision. This allows grading of the tumor and preparation for adjunctive medical therapies.
Limbal involvement may herald corneal epithelial and scleral invasion, therefore such tumors should be accompanied by excision of a thin lamella of sclera to achieve tumor-free margins (Shields JA, Arch Ophthalmol 1997).
Use of a microscopic technique with drying of the operative field (to help cell adherence to resected tissue) is advised (Shields CL et al., Oculoplastics and Orbit, Springer 2006).
Mohs surgical excision can be considered as a surgical option (Buus DR, Am J Ophthalmol 1994) particularly for recurrent cases.
Cryotherapy induces ischemic necrosis and can be used as an adjunctive treatment to excisional surgery with a recommended double freeze-thaw technique. It can be used as a primary treatment for primary acquired melanoma and pagetoid spread of sebaceous gland carcinoma. Care should be taken regarding length of placement of the probe and location; both extensive surgical excision and cryotherapy can cause limbal stem cell insufficiency.
Technique of wide local excision:
- 3-4 mm-wide surgical margins marked
- Surgical excision with no-touch
- Specimen oriented in consultation with pathologist for margin control
- Absolute alcohol to involved areas of cornea for 1 minute (corneal epitheliectomy) and irrigation with saline
- Corneal cells debrided (maintain intact Bowman’s layer) and sent for evaluation
- Cryotherapy (double freeze-thaw) to surgical margins (conjunctiva is elevated from underlying sclera to reduce damage)
- Amniotic membrane graft sutured into place +/- symblepharon ring
- Other options include direct closure for small defects or use of allografts (buccal mucosal, etc.).
Sentinel lymph node biopsy has not yet been investigated in the setting of OSSN and invasive SCC.
Modified enucleation with complete removal of all affected conjunctiva intact with the globe may be required in certain cases.
Eyelid-sparing or sacrificing orbital exenteration may be required in advanced cases that show orbital invasion, complete conjunctival involvement, or failure of response to other treatment modalities.
Other management considerations
Margin control through excision and adjunctive chemotherapy/radiotherapy remain the most popular modalities of treatment.
Frozen section control can potentially be as successful as Mohs surgery, although formal investigation is awaited. Specimen handling is critical during surgery with direct communication with the pathologist.
Common treatment responses, follow-up strategies
With the variation in recurrence rates in different studies pertaining to follow-up periods, severity of OSSN, and treatment modalities used, follow-up every 4–6 months for at least 3 years would be recommended.
Metastatic routes include direct extension, lymphatic, and hematogenous pathways, although this is rarer compared to conjunctival melanoma.
Preventing and managing treatment complications
Complications of surgery: symblepharon, nonhealing epithelial defects, hyphema, limbal stem cell deficiency, corneal scarring, infection, diplopia. Amniotic membrane grafts are useful in preventing many of these complications.
Complications of cryotherapy: damage to conjunctiva, cornea, iris, conjunctival chemosis, subconjunctival hemorrhage, sclera/corneal tissue loss, ciliary body damage and hypotony, damage to eyelids, uvea, extraocular muscles, dry eyes, sclera melt. Ideally, best way to prevent these complication is to ensure one is not aggressive with cryotherapy, margins are preferentially treated and the conjunctiva is lifted away from the sclera/cornea during treatment. (Shields CL et al, Oculoplastics and Orbit, Springer 2006 ;Shields JA, Arch Ophthalmol 1997).
Complications of topical chemotherapy such as mitomycin include keratopathy, limbal stem cell disease, punctual stenosis and persistent corneal erosions. Caution and close observation are recommended during treatment.(Ditta, et al. Ophthalmology, 2011). 5-FU and interferon a2b have a better safety profile (Nanji AA, Curr Opin Ophthalmol 2013).
Complications of radiotherapy includes dry eyes, loss of lashes, focal cataracts, limbal stem cell deficiency, keratinization of conjunctiva and other corneal complications (Shields CL et al, Oculoplastics and Orbit, Springer 2006).
Disease-related complications
- Li (2015) performed a follow-up study on 43 patients with conjunctival dysplasia and squamous cell carcinoma, treated with excision and cryotherapy.
- There were three recurrences, all evident within one year.
- No additional recurrences became evident within five years.
- Besley (2014) performed a follow-up study on 135 patients treated topically with topical mitomycin C (0.4 mg/mL), interferon alpha-2b (1 million units/mL) or both.
- There were 19 (14%) recurrences, of which 14 were evident within two years.
- Mean time to recurrence was 17 months but the latest recurrence was at 61 months.
- There was no increased risk of recurrence with treatment type.
- Vision damage or loss
- Local invasion into ocular adnexal structures including eyelids, cornea, sclera extension, nasolacrimal duct, orbital extension, sinus, CNS extension
- Metastatic disease to regional lymph nodes, then other organs
- Death (usually through metastasis related to a poorly differentiated tumor, large tumor size, orbital involvement at the time of exenteration, and high proliferation index (McKelvie P, BJO 2002).
Historical perspective
The first case of conjunctival squamous lesion was described in 1860 by Von Graefes (Duke Elder S, Systems of Ophth 1985).
In 1978, Pizzarello and Jakobiec classified CIN as mild, moderate and severe dysplasia based on the extent of epithelial involvement (Pizarello et al., Ocular Adnexal Tumors 1978).
In 1995, Lee and Hirst coined the term OSSN (Lee GA et al., Surv Ophthalmol 1995).
References and additional resources
- Alves LF, Incidence of epithelial lesions of the conjunctiva in a review of 12,102 specimens in Canada (Quebec). Arq Bras Oftalmol. 2011;74(1):21-3
- Amin MB, Edge S, Greene F, et al., eds. AJCC Cancer Staging Manual. 8th ed. Chicago; Springer International Publishing: 2017.
- Arepalli S, Plaque radiotherapy in the management of scleral-invasive conjunctival squamous cell carcinoma: an analysis of 15 eyes. JAMA Ophthalmol. 2014 Jun;132(6):691-6.
- Barbazetto IA, Lee TC, Abramson DH. Treatment of conjunctival squamous cell carcinoma with photodynamic therapy. Am. J. Ophthalmol. 138(2), 183–189 (2004).
- Basti S, Macsai MS. Ocular surface squamous neoplasia: a review. Cornea. 2003;22(7):687-704.
- Besley J, Pappalardo J, Lee GA, et al. Risk factors for ocular surface squamous neoplasia recurrence after treatment with topical mitomycin C and interferon alpha-2b. Am J Ophthalmol 2014; 157:287-293.
- Buus DR. Microscopically controlled excision of conjunctival squamous cell carcinoma. Am J Ophthalmol. 1994 Jan 15;117(1):97-102.
- Ditta LC et al. Outcomes in 15 patients with conjunctival melanoma treated with adjuvant topical mitomycin C: complications and recurrences. Ophthalmology, 2011; 118(9), pp. 1754-1759.
- Duke-Elder S, Leigh AG. Diseases of the outer eye.In: Duke-Elder S, ed. Systems of Ophthalmology, Vol 7, Part 2. St Louis: CV Mosby, 1985:1154–1159.
- Edge SB, Byrd DR, Carducci M, Compton CC (eds) AJCC Cancer Staging Manual. 7th edn. Springer: New York, 2009, pp 531–538
- Falke K. Diagnosis of conjunctival neoplastic lesions by confocal in-vivo microscopy. Klin Monbl Augenheilkd. 2012 Jul;229(7):724-7
- Faramarzi A, Feizi S. Subconjunctival bevacizumab injection for ocular surface squamous neoplasia. Cornea 2013;32:998–1001
- Gichuhi S et al. Pathophysiology of ocular surface squamous neoplasia. Experimental Eye Research 129(2014) 172-182
- Greenfield JA, Cohen AK, Galor A, Chodosh J, Stone D, Karp CL. Ocular Surface Squamous Neoplasia: Changes in the Standard of Care 2003 to 2022. Cornea. 2024;43(8):942-949.
- Gupta S et al. Ocular Surface Squamous Neoplasia. Delhi Journal of Ophth. Vol 23, No 2, October-December 2012
- Hirst LW. Randomized controlled trial of topical mitomycin C for ocular surface squamous neoplasia. Ophthalmology 2007; 114:976.
- Kim HJ, Shields CL, Shah SU, Kaliki S, Lally SE. Giant ocular surface squamous neoplasia managed with interferon ?-2b as immunotherapy or immunoreduction. Ophthalmology 119(5), 938–944 (2012)
- Kounatidou NE, Vitkos E, Palioura S. Ocular surface squamous neoplasia: Update on genetics, epigenetics and opportunities for targeted therapy. Ocul Surf. 2025;35:1-14.
- Krachmer JH,Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnosis and Management. Vol 1. Philadelphia, PA: Mosby Elsevier; 2005
- Krilis M, Tsang H, Coroneo M. Treatment of conjunctival and corneal epithelial & neoplasia with retinoic acid and topical interferon alfa-2b: long-term follow-up. Ophthalmology 2012; 119:1969 – 1973.
- Lecuona et al. The treatment of carcinoma in situ and squamous cell carcinoma of the conjunctiva with fractionated strontium-90 radiation in a population with a high prevalence of HIV Br J Ophthalmol 2015;99:1158-1161
- Lee GA, Hirst LW. Incidence of ocular surface epithelial dysplasia in metropolitan Brisbane. A 10-year survey. Arch Ophthalmol. 1992;110(4):525-7.
- Lee GA, Hirst LW. Ocular surface squamous neo- plasia. Surv Ophthalmol 1995; 39:429–50
- Lee, G.A., Hirst, L.W., 1997. Retrospective study of ocular surface squamous neoplasia. Aust. N. Z. J. Ophthalmol. 25,
- Li AS, Shih CY, Rosen L, et al. Recurrence of ocular surface squamous neoplasia treated with excisional biopsy and cryotherapy. Am J Ophthalmol 2015; 160:213-219.
- Margo CE, Mack W, Guffey JM. Squamous cell carcinoma of the conjunctiva and human immunodeficiency virus infection. Arch Ophthalmol 1996; 114:349
- McKelvie P et al. Squamous cell carcinoma of the conjunctiva: a series of 26 cases. Br J Ophthalmol 2002;86:168-173
- Monroy D, Serrano A, Galor A, Karp CL. Medical treatment for ocular surface squamous neoplasia. Eye (Lond). 2023;37(5):885-893.
- Nanji AA et al. Topical chemotherapy for ocular surface squamous neoplasia. Curr Opinion Ophth, Vol 24, No.4, July 2013 336-342
- Newton R, Reeves G, Beral V, et al. Effect of ambient solar ultraviolet radiation on incidence of squamous-cell carcinoma of the eye. The Lancet 1996;347:1450–1451
- Osahon, Adesuwa I., et al. Asian Pac J Trop Biomed. 2011 Apr; 1(2): 150–153
- Özcan AA, Çilo?lu E, Esen E, ?imdivar GH. Use of topical bevacizumab for conjunctival intraepithelial neoplasia. Cornea 2014;33:1205–1209
- Parrozzani R. In vivo confocal microscopy of ocular surface squamous neoplasia. Eye (Lond). 2011 Apr;25(4):455-60.
- Pe’er J. Ocular surface squamous neoplasia. Ophthalmol Clin North Am. 2005;18(1):1-13,vii
- Pizzarello LD, Jakobiec FA. Bowen’s disease of the conjunctiva: a misomer. In: Jakobiec FA, ed. Ocular Adnexal Tumors, Birmingham, AL: Aescula- pius, 1978:553–571.
- Rankin JK et al. An improved approach to diagnosing and treating conjunctival mucoepidermoid carcinoma. Surv of Ophth Vol 57, No.4, Jul-Aug 2012, 337-346
- Shah SU. Topical interferon alfa-2b for management of ocular surface squamous neoplasia in 23 cases: outcomes based on American Joint Committee on Cancer classification. Arch Ophthalmol. 2012 Feb;130(2):159-64
- Shields CL, Demirci H, Karatza E, Shields JA. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology. 2004;111(9):1747-54. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian J Ophthalmol. 2019;67(12):1930-1948.
- Shields CL, Shields JA, Chapter 2 – current concepts in the management of conjunctival neoplasms. Essentials in Ophthalmology: Oculoplastics and Orbit, Springer 2006)
- Shields JA. Surgical management of conjunctival tumors. The 1994 Lynn B. McMahan Lecture. Arch Ophthalmol. 1997 Jun;115(6):808-15.
- Shousha MA. Diagnosis and management of conjunctival and corneal intraepithelial neoplasia using ultra high-resolution optical coherence tomography. Ophthalmology. 2011 Aug;118(8):1531-7
- Spinak M, Squamous cell carcinoma of the conjunctiva. Value of exfoliative cytology in diagnosis. Surv Ophthalmol.1977 Jan-Feb;21(4):351-5.
- Tsatsos M,Karp C, Modern Management of Ocular Surface Squamous Neoplasia. Expert Rev Ophthalmol. 2013;8(3):287-295.
- Waddell, K.M., Downing, R.G., Lucas, S.B., Newton, R., 2006. Corneo-conjunctival carcinoma in Uganda. Eye (Lond.) 20, 893-899
- Xu Y et al. The clinical value of in vivo confocal microscopy for diagnosis of ocular surface squamous neoplasia. Eye (Lond). 2012 Jun;26(6):781-7
Financial disclosures
Reviewers
Dianne Schlachter – Consultant & Speaker, Amgen
