Reconstructing Large Defects Involving Forehead, Cheeks
Updated May 2024
Goals, indications, contraindications
Goals
- To repair the defect with the best possible functional and aesthetic outcome
Indications
- Repair of defects secondary to
- Excision of cutaneous neoplasm
- Trauma or burns
- Congenital causes
Contraindications
- Patients who are poor candidates for surgery due to underlying medical concerns
Preprocedure evaluation
Patient history
- Past medical history
- Previous history of skin cancer of the head and neck
- Underlying diagnosis that led to tissue defect
- Current medications
- Particular attention to anticoagulants, and nutritional supplements that affect bleeding
- Allergies
- Ensure negative margins if secondary to excision of cutaneous neoplasm
- History of diabetes or smoking, that make affect healing process
Clinical exam
- Measure the size of the defect and which aesthetic units are involved.
- Evaluate the depth of the defect.
- If the eyelid is involved, determine whether posterior lamella is intact.
- Evaluate preoperative eyelid position.
- Lagophthalmos
- Complete ophthalmic exam
Preoperative assessment
- Evaluate skin redundancy near the defect.
- Look for previous scars or natural skin tension lines in which the scar can be hidden.
- Determine the preferred technique to close the defect.
- The choice should result in the best possible match in skin color and texture.
- Horizontal tension should be maximized and vertical tension minimized to prevent eyelid retraction.
- Ideally, adjacent flaps are preferred over skin grafts due to optimal blood supply and skin match.
- Helpful to think of the periocular area in aesthetic units:
- Aesthetic units include the upper and lower eyelids, medial canthus, nose, lateral canthus, and cheek, and eyebrows, glabella, and forehead.
- Defects that cross several aesthetic units should be addressed separately to avoid abnormal scarring or webbing.
- Take preoperative photographs with angles that best show the defect — usually full-face frontal view and either oblique or lateral view.
Procedure alternatives
Small defects can be allowed to heal by secondary intention (Lowry, OPRS, 1997), although the cosmetic and functional outcome may be suboptimal.
In patients who are not good candidates for surgery, large cutaneous neoplasms may be treated medically.
Surgical techniques
Direct closure
(Soliman, Plast Reconstr Surg 2011; Rapstine, Plast Reconstr Surg 2012)
- Usually not an option for larger defects
- Minimize vertical tension when near the eyelid.
- Close the deep layer with buried absorbable sutures, typically 4-0 or 5-0 polyglactin (Vicryl) or poliglecaprone 25 (Monocryl) suture.
- Skin can be closed with 5-0 or 6-0 polypropylene, nylon, or plain gut absorbable suture.
- Conservatively excise Burow’s triangles at the end of the closure if a standing “dog-ear” deformity is present.
Local flaps
Advancement
(Patrinely, Surv Ophthalmol 1987; Hayano, J Skin Cancer 2012; Patel, Op Techniq Otolaryngol 2012; Lee, OPRS 2012)
- Meticulous dissection in the appropriate plane is crucial to avoid damage to the facial nerve.
- Involves the advancement of surrounding tissue along a linear axis to close the defect.
- Length-to-width typically a ratio of 3:1 to avoid tip necrosis. Longer flaps of 4:1 can be created if based along a named larger artery.
- Often creates “dog ear” deformities, which must be addressed as part of the reconstruction plan. Dog ear deformity occurs when one side of an incision has more tissue than the other side. To correct the problem, excess skin must be excised from the side with excess skin. Options to repair these iatrogenic deformities include
- Use of Burow’s triangle (triangular wedge of tissue excised from side with excess skin)
- Use of a hockey stick or elliptical incision
- Lengthening the defect to ensure similar tissue quantity on both sides of the incision
- A to T, V to Y, and island pedicle flaps are examples of advancement flaps. Island flaps maintain a subcutaneous pedicle from the original flap, which is transferred to the defect.
- A to T/O to T (T-plasty):
- Transforms an A- or O-shaped defect into a T-shaped scar by advancing 2 flaps on opposite sides of the defect toward each other
- Useful for the forehead, lip, and chin
- V to Y:
- V-shaped flap is moved into the defect and the secondary triangular defect is closed by approximating the 2 wound edges, creating a Y-shaped scar.
- Useful for the medial canthus, forehead, and nose
- Can be used in the cheek, but care should be taken to avoid vertical tension and resulting ectropion of the lower lid
- Island pedicle flap:
- Recruits skin and subcutaneous tissues with a reliable vascular supply
- Allows for larger flaps with good viability
- Useful for the cheek, eyebrow, nose, and medial canthus (Figures 1–3)
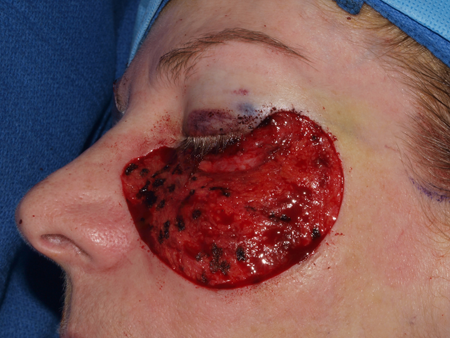
Figure 1. Left cheek and lower eyelid defect measuring 7.5 cm x 5 cm after excision of melanoma in situ.
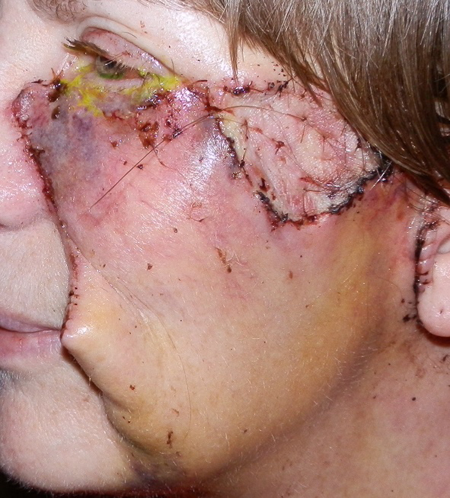
Figure 2. One week after reconstruction with cervicofacial advancement flap, canthoplasty, and full-thickness skin grafts to left lower eyelid and temple area to cover secondary defects. Note the standing cutaneous deformity near the left oral commissure.
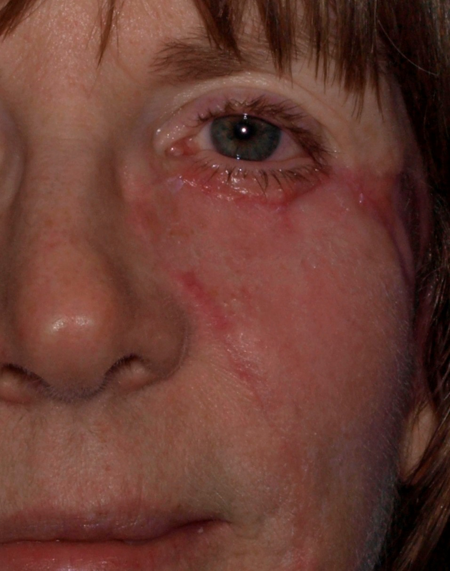
Figure 3. Two months after second stage procedure with removal of the standing cutaneous deformity.
Rotational
(Patel, Op Techniq Otolaryngol 2012; Ebrahimi, J Craniofac Surg 2013)
- Good tissue match
- Require preoperative planning to minimize secondary deformities
- Pivoted around a fixed point at the base of the flap, and rotated on an arc toward the defect
- Rotated on an arc of 30º or less with the radius 2–3 times the diameter of the defect, and the arc length approximately four to five times the width of the defect
- To minimize standing cutaneous deformity, closure of a triangular defect should have 2:1 height to width ratio.
- Examples include O to Z, cervicofacial, and glabellar flaps (Figures 4–6). O to Z:
- Double rotational flap at opposing sides of a circular or oval defect
- Pedicles are created 180º from each other.
- Each flap is advanced, rotated, and fixed 90º from its incision point.
- Flaps can be equal or unequal length depending on surrounding tissue, but closure tension is least with equal flap lengths and angle.
- Converts a circular defect into a Z shaped closure
- Useful for defects of the forehead, scalp, eyelid, and cheek — where tissue is available on both sides of the defect
- Cervicofacial:
- Extended form of the Mustarde flap
- Facial skin lateral and inferior to the defect is rotated in to allow closure.
- Incision begins at lateral superior edge of defect, proceeds down the preauricular skin, and then inferiorly into the neck.
- Due to the size of the flap, helpful to include platysma in the flap for improved vascularity
- Useful for large defects of the cheek, temple, and lower eyelid
- When used in the cheek and lower lid, often requires concurrent stabilization of the lower lid with canthoplasty
- Glabellar:
- Transfers skin from the glabellar region to cover defects of the side of the nose and medial canthal region
- Taken from hairless area between eyebrows and adjacent forehead, pivoting around the superior orbital foramen on the opposite side from the defect
- Triangular flap with apex pointing upwards, secondary triangular defect is closed by approximating wound edges.
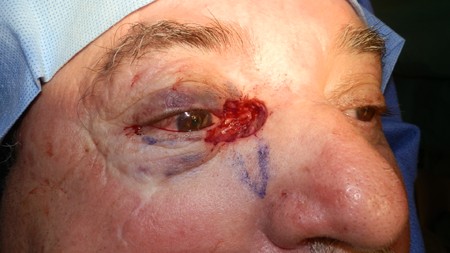
Figure 4.
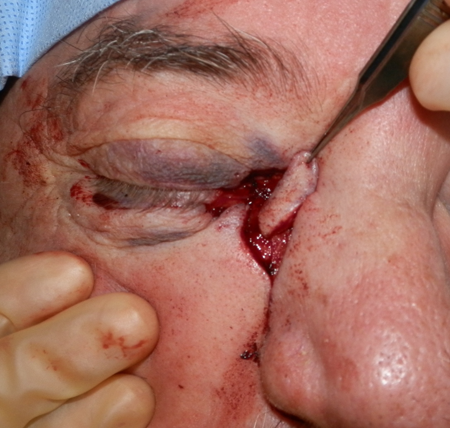
Figure 5.
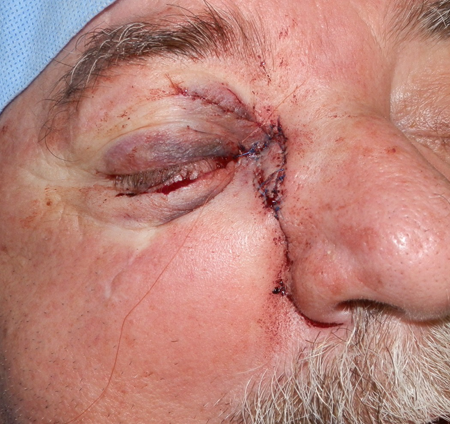
Figure 6.
Transpositional
(Patel, Op Techniq Otolaryngol 2012; Ibrahim, J Craniofac Surg 2012)
- Versatile flaps that create a secondary defect
- Flap is raised from a donor site over an incomplete bridge of skin to be placed into the defect site.
- The donor site must also be closed as part of the design.
- Examples include rhombic, bilobed, and Z-plasty.
- Rhombic:
- Square flap tilted toward one side, used to repair defects with rhombus or diamond shape
- Transfer involves advancement of tissue with a pivot point.
- Classic description by Limberg has two 60º angles and two 120º angles: 2 equilateral triangles placed base to base.
- Can be modified as needed to fit size of defect
- Most tension is at closure of donor site.
- Up to 4 rhombic flaps can be designed for a defect.
- Tissue extensibility and surrounding mobile structures should be taken into account when designing flap.
- Useful for the cheek and temple
- More difficult to hide this scar in relaxed tension lines
- Bilobed:
- Double transposition flaps that share a common base
- The greater the arc of rotation, the greater the standing cutaneous deformity
- Linear axis of each lobe should not be more than 45º from each other.
- The central lobe is moved in to fill the primary defect, then the second smaller lobe is moved in to fill the secondary defect.
- Advantage is recruitment of redundant skin from area that is not adjacent to the defect.
- Useful for the nose and the cheek
- Z-plasty:
- Double transposition of triangular flaps with independent pivot points
- 3 arms of Z should be same length.
- 60º angles are adequate for most repairs.
- Useful for scar revision with the scar positioned in the central limb of the Z
- Useful on forehead, cheek, lids, and for medial canthal webbing
- Multiple adjacent z-plasties can be used for larger scars.
Interpolated
(Patel, Op Techniq Otolaryngol 2012; Kim, Arch Plast Surg 2013)
- Pedicled flap that cross over or under intervening intact tissue
- The flap must be divided in a second stage of reconstruction.
- In contrast to transposition flaps, the base of interpolated flaps is not contiguous with the defect base.
- Examples include paramedian forehead and melolabial flaps, although the paramedian flap can be modified by removing the epidermis from the segment that passes under the bridge thereby making it a one-stage reconstruction without the need for later separation.
Free skin graft
(Patel, Op Techniq Otolaryngol 2012; Angelos, Fac Plast Surg Clin North Am 2013)
- Match like-tissue to like-tissue.
- If possible, avoid placing hair-baring skin in locations that do not usually have hair.
- May cause color mismatch and skin surface irregularity.
- Frequent graft contracture may occur. When placed on the lower lid, a temporary Frost tarsorrhaphy to elevate the lower lid and graft upwards may be useful in minimizing contracture.
- Small venting incisions within the graft to allow egress of serosanguinous fluid can help prevent graft failure if bleeding occurs.
Microvascular free flap
(Patel, Op Techniq Otolaryngol 2012; Thorwath, Oral Maxillofac Surg, 2008; Angelos, Fac Plast Surg Clin North Am 2013)
- Time intensive
- Requires advanced techniques. Must have anastomosis donor arterial and venous supply to host site vasculature.
- Typically taken from the radial forearm (radial artery) or anterolateral thigh (descending branch of the lateral circumflex femoral artery)
- Often a poor match in skin color and texture
Tissue expansion
(Cannon, OPRS 2011)
- Primarily used in breast and burn reconstruction
- By increasing the surface area of tissue adjacent to a defect, tissue expanders enable repair by local tissue transfer.
- Once tissue expander is placed, saline is used to inflate the expander. Typically inject 1–2 cc of saline each 1–2 weeks. May take 3–4 months to achieve adequate expansion. Be careful not to overinflate, or may cause overlying skin necrosis.
- 35–60% increased skin surface area created.
- Once adequate expansion is created, the defect can be closed with rotational or transposition flap.
- Requires multiple surgical procedures and clinic visits
Acellular human dermal matrix
(AlloDerm, LifeCell, Bridgewater, NJ) (Levin, OPRS 2011)
- Useful adjunct for large periocular defects in patients who are not good candidates for skin grafts or local flaps due to comorbid conditions
- Allows for epithelium to grow over the dermal matrix
Negative pressure wound therapy
(Semlacher, OPRS 2012)
- NPWT at 75 mm Hg can expedite wound healing and improve skin graft survival.
- Has been shown in case report to be safe in the periocular area, with no long-term effect on vision or intraocular pressure (IOP). IOP should be monitored while on NPWT.
Hatchet flap
- This technique is useful in the setting of large medial mid-facial defects.
- An incision is created along the medial, inferior aspect of the defect into the nasolabial fold.
- Laterally, a small transposition flap is created along the nasal ala.
- The flap is undermined to the level of the defect, rotated superiorly, and anchored with sutures.
Patient management: treatment and follow-up
Postoperative instructions
- Apply antibiotic ointment to the surgical incisions several times a day for a week.
- No ice or heat should be applied to large flaps and skin grafts.
- Strenuous activity and bending should be avoided for the first few weeks.
- Patients should not smoke after surgery.
Medications prescribed
- Topical ophthalmic antibiotic ointment for incisions near the eye (erythromycin or bacitracin ophthalmic ointment)
- Topical antibiotic ointment for other incisions on the face and scalp
- Oral antibiotic (Keflex, or Clindamycin if penicillin allergic)
- Oral pain medication as needed
Other management considerations
- Patients should be followed closely the first few weeks postoperatively to monitor for signs of infection, wound dehiscence, hematoma formation, and flap necrosis.
- The eyelids, canthal angle, and eyebrows are mobile. They are therefore vulnerable to malposition caused by wound bed contraction.
- For large flaps, a drain may be placed to avoid hematoma formation. This can usually be removed the first postoperative day, or when drainage is nominal.
- In patients without a drain, a pressure bandage for 24–48 hours can help prevent hematoma formation.
- Patients return for suture removal 7 to 10 days after surgery.
- In patients with previous radiotherapy, all attempts should be made to close the defect with a vascularized flap. Irradiated tissue typically has very poor vascular supply, greatly increasing the risk of graft failure.
Preventing and managing treatment complications
Hematoma formation
- May compromise flap viability if not treated early
- Prevent with meticulous hemostasis intraoperatively, and avoidance of anticoagulants postoperatively.
- Venting incisions in large skin grafts, and a pressure patch/bolster initially after surgery can minimize bleeding under the graft.
- If a small hematoma develops (< 10 ml), may aspirate with 25 cc syringe and 18 gauge needle placed through the incision.
- For large hematomas, best to return to OR to open incision, drain hematoma, and control active bleeding.
Infection
- Excessive pain and erythema at the incision site 4–8 days after surgery
- Can result in poor outcome due to distortion from associated edema
- Staphylococcus aureus most common etiology
- Risk factors include delayed repair and patients with diabetes or immunosuppression.
- In these cases, consider intraoperative intravenous cephalosporin, followed by 7-10 days of oral cephalosporin.
- If infection does develop, culture the wound, and tailor antibiotics to results. Any abscess should be promptly drained.
Flap ischemia
- Minimize by understanding vascular anatomy, and basing the flap off existing vasculature. If random pattern flap is used, dissection should be in the subcutaneous tissue to ensure an intact subdermal plexus.
- Risk factors include excessive flap thinning, aggressive cautery, crush injury from instruments, and excessive tension on the wound during closure.
- Aggressively treat any underlying cause such as hematoma or infection.
- Topical vasodilators, such as 2% nitroglycerin ointment to flap, may be of benefit.
- If necrosis develops, debride that portion of the flap. May allow to heal by secondary intention.
Wound contracture
- More common with free skin grafts
- Oversize the graft to account for contraction
Eyelid/eyebrow malposition
- Can be minimized at the time of surgery with concurrent canthoplasty and deep fixation sutures that secure the flap to the underlying periosteum
- May need second surgical procedure to correct the malposition
Scar formation
- Hypertrophic scarring more common in patients with darker skin and those with a family history of hypertrophic scar formation
- Minimize scar formation with UV protection (sunglasses, sunscreen), vitamin E oil, or silicone sheeting.
- Can treat existing scars with dermabrasion (abrade until pinpoint bleeding), intralesional injection of 5-fluorouracil and Kenalog (typically 9:1 ratio of 50mg/cc 5 fu and 40mg/ml Kenalog), or surgical revision.
Disease-related complications
- Local or metastatic recurrence of the neoplastic process originally resected
Historical perspective
- The Mustarde flap, originally described in 1970, is a rotation advancement flap used classically for the repair of defects of the cheek, temple, and lower eyelid.
- Contemporary flaps, such as the cervicofacial advancement flap, are extended versions of the Mustarde flap.
References and additional resources
- AAO, Basic and Clinical Science Course. Section 7: Orbit, Eyelids and Lacrimal System, 2011-12
- Angelos PC, Downs BW. Options for the management of forehead and scalp defects. Facial Plast Surg Clin North Am. 2009 Aug;17(3):379-93
- Baker, SR, ed. Local Flaps in Facial Reconstruction, 2nd ed. Philadelphia: Elsevier; 2007
- Cannon PS, McCormick A, Leatherbarrow B. The use of soft tissue expanders in periocular reconstruction: a case series. Ophthal Plast Reconstr Surg. 2011 Jul-Aug;27(4):232-5
- Custer PL and Maamari RN. Hatchet flap with transposed nasal inset for midfacial reconstruction. Ophthal Plast Reconstr Surg, 2018; 34:393-5
- Ebrahimi A, Nejadsarvari N. Experience with cervicofacial flap in cheek reconstruction. J Craniofac Surg. 2013 Jul;24(4):e372-4
- Harris, GH. Atlas of Oculofacial Reconstruction: Principles and Techniques for Repair of Periocular Defects. Philadelphia: Lippincott Williams & Wilkins; 2009
- Hayano SM, Whipple KM, Korn BS, Kikkawa DO. Principles of Periocular Reconstruction following Excision of Cutaneous Malignancy. J Skin Cancer. 2012;2012:438502
- Ibrahim AM, Rabie AN, Borud L, et al. Common patterns of reconstruction for Mohs defects in the head and neck. J Craniofac Surg. 2014 Jan;25(1):87-92
- Kim JH, Kim JM, Park JW, et al. Reconstruction of the medial canthus using an ipsilateral paramedian forehead flap. Arch Plast Surg. 2013 Nov;40(6):742-7
- Lee BJ, Elner SG, Douglas RS, Elner VM. Island pedicle and horizontal advancement cheek flaps for medial canthal reconstruction. Ophthal Plast Reconstr Surg. 2011 Sep-Oct;27(5):376-9
- Levin F, Turbin RE, Langer PD. Acellular human dermal matrix as a skin substitute for reconstruction of large periocular cutaneous defects. Ophthal Plast Reconstr Surg. 2011 Jan-Feb;27(1):44-7
- Lowry JC, Bartley GB, Garrity JA. The role of second-intention healing in periocular reconstruction. Ophthal Plast Reconstr Surg. 1997: 13:174-88.
- Patel KG, Sykes JM. Concepts in local flap design and classification. Operative Techniques in Otolaryngology. 2011;22: 13-23
- Patrinely JR, Marines HM, Anderson RL. Skin flaps in periorbital reconstruction. Surv Ophthalmol 1987;31(4):249-61
- Rapstine ED, Knaus WJ 2nd, Thornton JF. Simplifying cheek reconstruction: a review of over 400 cases. Plast Reconstr Surg. 2012 Jun;129(6):1291-9
- Semlacher RA, Taylor EJ, Golas L, et al. Safety of negative-pressure wound therapy over ocular structures. Ophthal Plast Reconstr Surg. 2012 Jul-Aug;28(4):e98-101
- Soliman S, Hatef DA, Hollier LH, Thornton JF. The rational for direct linear closure of facial Mohs’ defects. Plat Reconstr Surg. 2011 Jan;127(1):142-9
- Thorwarth M, Eulzer C, Bader R, et al. Free flap transfer in cranio-maxillofacial surgery: a review of the current data. Oral Maxillofac Surg. 2008 Sep;12(3):113-24
