Idiopathic Orbital Inflammation
Updated May 2025
Establishing the diagnosis
Etiology
Idiopathic orbital inflammation (IOI) is by definition idiopathic, although sometimes it is associated with systemic disease, such as inflammatory bowel disease or recent upper respiratory infection in children.
Epidemiology
IOI occurs in all ages, men and women (mean age 45 years old).
IOI is the second most common cause of proptosis following thyroid eye disease (TED).
IOI is the third most common orbital disorder following TED and lymphoproliferative disease, accounting for 5–17.6% of orbital disorders.
Pediatric cases account for about 17% of IOI cases.
History
The most common pattern of presentation is sudden or subacute onset of pain and periorbital swelling.
Decreased vision or double vision is possible, depending on location of inflammation.
Unilateral involvement is more common (26% bilateral in Yuen 2003).
Clinical features
IOI is a noninfective clinical syndrome characterized by features of nonspecific inflammatory conditions of the orbit, most often without identifiable local or systemic causes.
Anterior orbital inflammation/diffuse inflammation:
- Pain
- Proptosis
- Erythema and edema of lids
- Chemosis
- Decreased vision
- Decreased extraocular motility
- Uveitis/scleritis
- Choroidal folds (Figure 1)
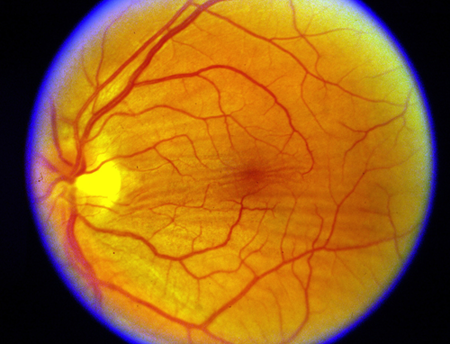
Figure 1. Choroidal folds.
Apical inflammation (Tolosa-Hunt syndrome):
- Pain
- Proptosis
- Decreased vision
- Decreased extraocular motility
Myositic inflammation:
- Pain worse with eye movement
- Decreased extraocular motility
- Localized chemosis with conjunctival injection
Lacrimal inflammation (dacryoadenitis):
- Pain
- S-shaped deformity of lid
- Localized chemosis with conjunctival injection
Sclerosing pseudotumor:
- Can have painless, gradual loss of orbital function
- Decreased vision
Testing
CT scan with fat suppression or MRI scan:
- Generally seen as an enhancing infiltrative mass with irregular margins
- Myositic: Fusiform enlargement of extraocular muscle involving the tendons of insertion (Figure 2)
- Lacrimal (dacryoadenitis, Figure 3): Enlargement of the lacrimal gland with some hazy involvement of adjacent fat (fat-stranding), bone intact
- Anterior: Enhancing infiltrative mass involving the optic sheath complex, uvea, sclera
- Diffuse: Multifocal intraconal enhancing infiltrative mass, with or without extraconal orbital involvement (Figure 4)
- Apical (Tolosa-Hunt syndrome): Enhancing infiltrative orbital apex, with variable intracranial involvement, typically at the cavernous sinus
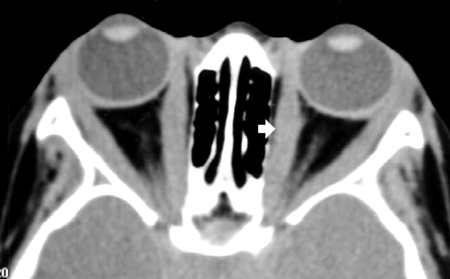
Figure 2. Orbital myositis.
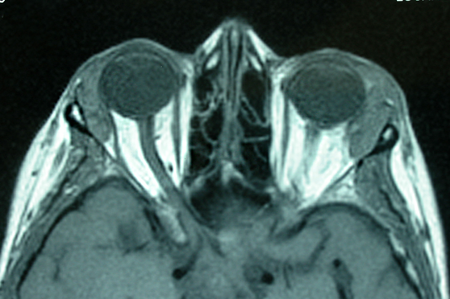
Figure 3. Bilateral dacryoadenitis.
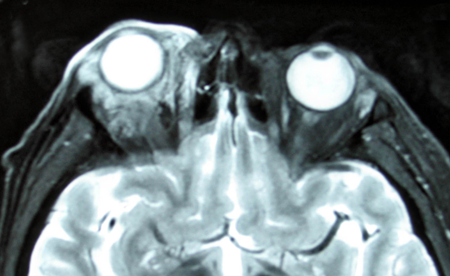
Figure 4. Diffuse IOI.
Two-phase helical CT and delayed coronal CT:
- These techniques can be useful in differentiating between orbital lymphoma and chronic or subacute idiopathic orbital inflammation.
- CT attenuation change over time was significantly different as described below (P < 0.05, Moon 2003).
- In lymphoma, there was increased CT attenuation between early- and late-phase axial scans followed by decline in CT attenuation between late-phase axial and delayed coronal scans.
- Conversely, in subacute idiopathic orbital inflammation, CT attenuation increased gradually over time from early-phase axial to delayed coronal scans.
Ultrasonography: Typical findings are reduced reflectivity, regular internal echoes, and weak attenuation, similar to lymphoproliferative lesions making ultrasound less useful for making a specific diagnosis.
Laboratory evaluation:
- WBC with differential: eosinophilia in IOI
- Erythrocyte sedimentation rate (ESR) is elevated in IOI.
- C-reactive protein (CRP) can be elevated in IOI.
- Antinuclear antibody (ANA) can be positive in IOI.
- Cerebrospinal fluid pleocytosis: mild elevation in IOI
- Rheumatoid factor (RF) can be positive in IOI.
- Angiotensin converting enzyme (ACE): High levels are indicative of sarcoidosis.
- Lysozyme: High levels are indicative of sarcoidosis.
- Antineutrophil cytoplasmic antibody (p-ANCA, c-ANCA) is diagnostic of polyangiitis with granulomatosis (Wegener).
- Serum IgG4
Testing for staging, fundamental impairment
Histopathology is nondiagnostic and variable:
- Polymorphous, nongranulomatous inflammation with eosinophils and infiltrative sclerosis
- Several classification schemes have been postulated, but none has been definitively accepted due to the absence of distinct differences among the histopathologic types as to the signs, symptoms, clinical course, and outcome.
- Orbital biopsy is primarily indicated to rule out specific nonidiopathic forms of inflammation or neoplastic disease.
- Microarray gene expression profiling, for genes commonly associated with inflammatory disease, does not enhance the diagnostic yield (Rosenbaum, 2017).
Risk factors
Few studies reported association with lower socioeconomic status, higher BMI, younger age at first child birth, use of oral bisphosphonates, and recent upper respiratory tract infection (Bijlsma 2011).
IOI has been reported in association with systemic autoimmune disorders, including Crohn’s disease, systemic lupus erythematosus, rheumatoid arthritis, diabetes mellitus, myasthenia gravis, ankylosing spondylitis, and others.
Differential diagnosis
Adults
Orbital cellulitis: Infection needs to be ruled out.
Orbital inflammation of specific etiology:
- Sarcoidosis (see Orbital and Ocular Adnexal Sarcoidosis section for more detail):
- Uveitis
- Dacryoadenitis
- Rare orbital lesions
- Tests:
- Angiotensin-converting enzyme
- Lysozyme
- Serum calcium
- Chest X-ray (hilar adenopathy)
- Positive gallium scan
- Polyangiitis with granulomatosis (see Granulomatosis with Polyangiitis section for more detail):
- Upper and lower respiratory tract lesions
- Vasculitis
- Nephritis
- Uveitis
- Orbital inflammation
- Tests:
- Antineutrophil cytoplasmic antibodies (p-ANCA, c-ANCA)
- Abnormal urinary sediment
- Abnormal chest x-ray (CXR)
- Sjögren syndrome:
- Dry eyes and mouth
- Other concurrent autoimmune diseases
- Enlarged lacrimal glands
- Tests:
- Antinuclear antibody (ANA)
- Sjögren antibodies
- Systemic lupus erythematosus:
- Characteristic skin lesions
- Facial rash, erythema
- Tests:
- ANA
- dsDNA, SSA, SSB
- Syphilis test: VDRL
- Ruptured dermoid cyst
- Arteriovenous malformation
- Orbital malignancy or metastasis
- Lymphoproliferative malignancies
- TED:
- Thyroid function testing:
- Thyroid stimulating hormone (TSH)
- Triiodothyronine (T3)
- Levothyroxine (T4)
- Thyroid antibodies: thyroid-binding inhibitory immunoglobulins (TBII), thyroid-stimulating immunoglobulins (TSI)
- Orbital CT or MRI scan: enlarged extraocular muscles with sparing of the tendons, and/or increased orbital fat volume
Children
- Orbital cellulitis
- Ruptured dermoid cyst
- Rhabdomyosarcoma
- Metastatic neuroblastoma
- Leukemic orbital infiltrate
- TED
Patient management: treatment and follow-up
Natural History
Clinical presentation varies depending on the involved tissues, ranging from a diffuse inflammatory process to a more localized inflammation of muscle, lacrimal gland, or orbital fat. Without treatment, fibrosis with loss of function of the involved tissues is expected.
Pediatric IOI
Pediatric IOI (17% of cases) can present slightly differently than in the adult population, including bilateral involvement.
- In 30 IOI patients younger than age 18, four (13%) had bilateral disease on presentation (Spindle, 2016).
- Dacryoadenitis (8 cases, 67%) was the most common presentation in 12 pediatric IOI patients, three (25%) had bilateral disease (Belanger, 2010).
- Uveitis, disc edema, and tissue eosinophilia are also more common in the pediatric population. Presence of uveitis generally implies a poorer outcome.
- Bilateral presentation might have a higher incidence of coexisting systemic disease.
- A recent case report highlighted the importance of early recognition and a comprehensive management strategy in pediatric IOI to improve outcomes. The case involved an eight-year-old female presenting with proptosis and visual disturbances, managed effectively with corticosteroids and multidisciplinary care (Elashmawy 2024).
Tolosa-Hunt Syndrome Variant of IOI
- Characterized by extension into the cavernous sinus through superior orbital fissure, with associated cranial nerve findings.
Sclerosing IOI
- This subtype often presents bilaterally and can have an elevated erythrocyte sedimentation rate (Winn 2012).
- Sclerosing IOI can be associated with systemic multifocal fibrosclerosis or other systemic inflammatory disease.
- Pemberton and Fay’s literature review showed no ethnicity, gender, or comorbidity predilection (Pemberton 2012).
- It typically presents in fourth decade of life with proptosis as most common feature (73%), pain (49%), normal vision (44%).
- It can extend into the sinuses.
- It is a recurrent or recalcitrant disease with no clear consensus on treatment regimen.
Medical Therapy
Oral Corticosteroids
- The mainstay of therapy.
- Clinicians often observe a dramatic response to oral corticosteroids within the first 24–48 hours, which has been viewed as pathognomonic for acute nonsclerosing IOI.
- The average prednisone starting dose is 60–80 mg/day.
- Gradually taper by 10–20 mg per week if tolerated without relapse of symptoms. The myositis subtype can require longer, slower taper (Yuen 2003).
- Closely monitor with medical doctor for side effects of steroids.
- Consider pulse-dosed intravenous dexamethasone prior to oral corticosteroids:
- 500 mg IV weekly for 6 weeks.
- 250 mg IV weekly for 6 weeks.
- About 37% patients treated with steroids show failure to resolve, in which case, consider biopsy.
- Empiric therapy can be effective if an idiopathic diagnosis is confident (tissue diagnosis not required).
Biopsy
- Indicated in cases of poor response to steroids, recurrent disease, or non-contributory serologic workup; many authorities advocate routine biopsy.
- Many surgeons choose to biopsy all infiltrative orbital lesions prior to subjecting a patient to long-term corticosteroid therapy. Exceptions include presumed orbital myositis or orbital apex lesions.
- If there is no improvement or the IOI recurs, consider performing an orbital biopsy.
Second-Line Pharmacologic Treatment Options
For steroid-intolerant or -resistant patients as part of a steroid-sparing strategy:
- Adjunctive NSAIDs: Indomethacin, Ketorolac.
- Mycophenolate Mofetil (Cellcept):
- Immunosuppressant and prodrug of mycophenolic acid.
- Purine nucleotide synthesis blocker that reversibly inhibits T and B lymphocyte proliferation.
- Antifibrotic effects.
- Common side effects include GI upset, abdominal pain, nausea, elevated cholesterol levels, hyperkalemia, hypocalcemia, coughing, and back pain.
- Increased infection risk, with leukopenia and anemia, reflecting the immunosuppressive and myelosuppressive effects of the drug on lymphocyte production.
- Typical starting dosage is 500 mg daily, increased to a maximum of 1.5–3 g daily.
- Work with a PCP or rheumatologist to monitor electrolytes, CBC, and serum lipids.
- Rituximab (Rituxan, MabThera, Zytux):
- A chimeric monoclonal antibody that targets protein CD-20, primarily found on the surface of B cells.
- Side effects include infusion site reactions, rash, rigors, fever, headache, infection, and bronchospasm.
- Ten patients received 500 mg or 1000 mg doses, intravenously twice on days 1 and 15, with reduction in steroid dependence and clinical inflammation at 24 weeks (Suhler, 2014).
- A recent case report demonstrated successful treatment of IOI with rituximab monotherapy, highlighting its potential as a third-line therapy (Bawazeer 2023).
- Infliximab (Remicade):
- An intravenous antibody that blocks the effects of tumor necrosis factor-alpha (TNF-alpha), thus decreasing signs and symptoms of inflammation.
- Risks include reactivation of tuberculosis and possible risk of lymphoma.
- Work with a PCP or rheumatologist to monitor electrolytes and CBC.
- Adalimumab (Humira):
- A recombinant IgG1 monoclonal antibody containing 100% human peptide sequences targeting TNF-alpha.
- Induction dose: 160 mg, then 80 mg, then 40 mg weekly.
- Risks include lymphoma (particularly in patients with long-standing rheumatoid arthritis), infection, and optic neuritis.
- Work with a PCP or rheumatologist to monitor electrolytes and CBC.
- Other Immunosuppressants: Methotrexate, Cyclosporine, Azathioprine.
- Cytotoxic Agents: Chlorambucil, Cyclophosphamide can be useful for sclerosing pseudotumor.
Radiation
- Radiation can be a primary treatment or an adjunctive option after medical therapy has been optimized.
- Studies have shown about 75% improvement with 2500 cGy over 15 days (Orcutt 1983) and benefits at doses of 2500–3000 cGy over 10 days (Kennerdell 1979) or 1000–2000 cGy over 10–15 days (Sergott 1981). There is a risk of developing a cataract at a dosage of 2000 cGy.
- Radiation can be less effective in fibrotic/sclerotic or granulomatous variants (compared to better response in myositis lesions) and in patients with a longer interval between diagnosis and treatment.
Surgery
- Biopsy is an option for diagnostic purposes, particularly if the IOI is not improved with steroid treatment.
- Complete excision of areas of involvement is usually not indicated or possible.
Recurrent dacryoadenitis may be managed with surgical debulking.
- In 46 patients with recurrent dacryoadenitis in whom steroid therapy was ineffective, treated with surgical debulking, the inflammatory process resolved within two months in 80% (37 of 46) (Mombaerts, 2014).
Surgical approach depends on area of orbital involvement; in general, the representative specimen(s) should be biopsied from an area with the least visual morbidity.
- Preoperative imaging with MRI or CT is necessary to guide the approach:
- Upper-lid crease incision approach:
- Mark the upper eyelid crease.
- Use local anesthesia and sedation.
- If lacrimal gland involvement is present, the orbital septum is incised laterally and the enlarged gland carefully biopsied from the orbital lobe anterior to the levator complex. Take care to avoid excision of the palpebral lobe because this risks injury to the excretory ducts that drain from the orbital to the palpebral lobe to exit the superior fornix.
- Orbital fat specimens or other involved tissue can also be biopsied if indicated.
- Inferior forniceal approach:
- Use local anesthesia and sedation.
- Perform a transconjunctival incision and blunt dissection to expose involved tissue and protect globe with Sewall or malleable retractors.
- After hemostasis, closure is usually not necessary.
- Other typical approaches include the transcaruncular incision or via lateral orbital incisions.
Fine-needle aspiration
- Not common practice with advent of other options
- Can be performed under CT guidance
- Shown to be able to differentiate lymphoma from orbital inflammatory lesions
Other management considerations
- In cases of extraorbital extension (about 9% of cases), biopsy is indicated to rule out infectious and neoplastic diseases.
- In bilateral cases in adults, lymphoma and thyroid dysfunction must be ruled out.
- Close collaboration with rheumatologist is often necessary.
- Strict medication compliance must be discussed with the patient, e.g., do not self-discontinue prednisone.
- Carefully taper the schedule of prednisone; it should be gradual enough to keep symptoms from flaring.
- Prescribe concurrent medications (antacids, proton pump inhibitors) for gastrointestinal protection during steroid use
Common treatment-responses, follow-up strategies
IOI typically responds quickly to prednisone, however, recurrent inflammatory episodes may require continued corticosteroid use.
- Additional treatment with steroid-sparing agents might be required.
- Dacryoadenitis may be particular recalcitrant to medical treatment – among 79 cases of biopsy proven idiopathic dacryoadenitis, 37% (29 0f 79) had recalcitrant or incomplete response to medical therapy (Andrew, 2016).
A clinical course could be more quiescent in patients who passed the critical period of the first year without developing systemic inflammatory diseases. Specific diseases tended to appear more often in diffuse forms of IOI (Maalouf 1999).
Preventing and managing treatment complications
Prednisone is the mainstay therapy.
Side effects:
- Inability to taper drug without flare of disease
- Hyperglycemia
- Aseptic necrosis of the hip
- Weight gain
- Cushingoid facies
- Depression
- Sleep difficulties
Prevention:
- Use prednisone at minimally effective dose.
- Limit long-term use.
- Discuss side effects with patient before use.
- Collaborate with primary care physician/rheumatologist.
Complications related to radiation therapy or cytotoxic agents
Because of potential for severe side effects, treatment must be individualized in collaboration with rheumatologist, and regular monitoring performed.
Disease-related complications
- Loss of vision
- Idiopathic sclerosing orbital inflammation (ISOI), a subtype of IOI, can cause orbital apex syndrome characterized by simultaneous vision loss and ophthalmoplegia. In a study, 42% of ISOI patients had orbital apex involvement, with some experiencing permanent visual deficits (Chang 2022).
- Diplopia
- A study of 52 patients with orbital myositis found that 49% presented with diplopia. The medial rectus was the most commonly involved muscle (58%), leading to abduction limitation in 73% of cases (Lasrado 2025).
- Six pediatric myositis patients developed acute restrictive strabismus with an enlarged extraocular muscle, with no known systemic cause (Sharma, 2016).
- Pediatric patients with acute restrictive strabismus and an enlarged extraocular muscle should be evaluated for recent streptococcal pharyngitis (Alshaikh, 2008).
- A case report described a 12-year-old girl with idiopathic lateral rectus myositis presenting with pain during horizontal eye movement. Imaging revealed isolated monolateral lateral rectus myositis, and the patient responded rapidly to systemic corticosteroids (Porhajihosseini 2022).
- Persistent/recurrent episodes of inflammation — need to rule out:
- Abscess/sequestrum
- Unusual organism
- Leaking dermoid
- Hematological malignancy
- Necrosis of solid tumor
- Noninfective vasculitis
- Recurrence in orbital myositis is associated with multiple muscle involvement and abrupt cessation of steroids. In a study, 43% of patients experienced recurrence, with abrupt steroid discontinuation being a significant risk factor (P = .046) (Lasrado 2025).
Historical perspective
- Idiopathic orbital inflammatory syndrome, also known as orbital pseudotumor, first described by Gleason in 1903 and by Busse and Hochhmein shortly after (Gleason 1903)
- Characterized as a distinct entity in 1905 by Birch-Hirschfeld
- Pediatric idiopathic orbital inflammation
- Alternatives to prednisone for treatment (nonsteroidal agents, cytotoxic, corticosteroid sparing immunosuppressants, IV immune-globin, plasmapheresis, radiation, biologic treatments)
- IgG4-related orbital inflammation (see IgG4-related orbital disease section)
- Presence of toll-like receptors in IOI first reported (Wladis 2012)
References and additional resources
- Adams AB, Kazim M, Lehman TJ. Treatment of orbital myositis with Adalimumab (Humira). J Rheumatol. 2005;13:1374-1375.
- Alshaikh M, Kakakios AM, Kemp AS: Orbital myositis following streptococcal pharyngitis. J Paediatr Child Health 2008; 44:233.
- Andrew NH, Kearney D, Sladden N, et al: Idiopathic dacryoadenitis: Clinical features, histopathology, and treatment outcomes. Am J Ophthalmol 2016; 163:148.
- Bawazeer, A., Rahali, W., Alsharif, A., Alshehri, M., Maksood, L., Babkier, A., Hommadi, W., Othman, B., Omair, M. A., & Hafiz, W. A. (2023). Idiopathic orbital inflammation treated with rituximab monotherapy. Cureus, 15(1), e33614.
- Belanger C, Zhang KS, Reddy AK, Yen MT, et al. Inflammatory disorders of the orbit in childhood: a case series. Am J Ophthalmol. 2010;150(4):460-463.
- Bijlsma WR, van Gils CH, Paridaens D, Mourits MP, et al. Risk factors for idiopathic orbital inflamation: A case-control study. Br J Ophthalmol. 2011;95:360–364.
- Chang, C. C., Chang, Y. C., Su, K. Y., Lee, Y. C., Chang, F. L., Li, M. H., Chen, Y. C., Chen, N. (2022). Acute orbital apex syndrome caused by idiopathic sclerosing orbital inflammation. Diagnostics, 12(12), 3003.
- Char DH1, Miller T. Orbital pseudotumor. Fine-needle aspiration biopsy and response to therapy. Ophthalmology. 1993;100(11):1702-1710.
- Elashmawy, A., Stephan, C., Shetty, A., Yangouyian, A., & Sharif, S. (2024). Idiopathic orbital inflammatory disease in a pediatric patient: A case report highlighting the diagnostic reasoning and treatment strategy. Cureus, 16(8), e67569.
- Garrity JA, Coleman AW, Matteson EL, Eggenberger ER, Waitzman DM. Treatment of recalcitrant idiopathic orbital inflammation (chronic orbital myositis) with Infliximab. Am J Ophthalmol. 2004;138:925-930.
- Gleason JE. Idiopathic myositis involving the extraocular muscles. Ophthalmol Rec. 1903;12:471-478.
- Griepentrog GJ, Vickers RW, Karesh JW, Azari AA, Albert DM, Burkat CN. A Clinicopathologic Case Study of Two Patients with Pediatric Orbital IgG4-Related Disease. Orbit. 2013;32:389-391.
- Hatton MP, Rubin PAD, Foster CS. Successful treatment of idiopathic orbital inflammation with mycophenolate mofetil. Am J Ophthalmol. 2005;140: 916-918.
- Ho VH, Chevez-Barrios P, Jorgensen JL, Silkis RZ, Esmaeli B. Receptor expression in orbital inflammatory syndromes and implications for targeted therapy. Tissue antigens. 2007;70(2):105-109.
- Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders. Am J Ophthalmol. 2000;130:492-513.
- Kapadia MK, Rubin PA. The emerging use of TNF-alpha inhibitors in orbital inflammatory disease. Int Ophthalmol Clin. 2006;46(2): 165-181.
- Kennerdell J, Johnson B, Deutsch M. Radiation treatment of orbital lymphoid hyperplasia. Ophthalmology. 1979;86:942-947.
- Lasrado, A. S., Chattannavar, G., Jakati, S., Mohamed, A., & Kekunnaya, R. (2025). Orbital myositis and strabismus: Clinical profile, management, and predictive factors for recurrence. Journal of Pediatric Ophthalmology and Strabismus, 62(1), 57–66.
- Maalouf T, Trouchaud-Michaud C, Angioi-Duprez K, George JL. What has become of our idiopathic inflammatory pseudo-tumors of the orbit? Orbit. 1999;18(3):157–166.
- Mombaerts I, Cameron JD, Chanlalit W, Garrity JA: Surgical debulking for idiopathic dacryoadenitis: A diagnosis and a cure. Ophthalmology. 2014; 121:603.
- Moon WJ, Na DG, Ryoo JW, et al. Orbital lymphoma and subacute or chronic inflammatory pseudotumor: differentiation with two-phase helical computed tomography. J Comput Assist Tomogr. 2003;27(4):510-516.
- Mottow-Lippa L, Jakobiec FA, Smith M. Idiopathic inflammatory orbital pseudotumor in childhood II. Results of diagnostic tests and biopsies. Ophthalmol. 1981;88(6): 565-574.
- On A, Hirschbien M, Williams H, Karesh J. CyberKnife radiosurgery and rituximab in the successful management of sclerosing idiopathic orbital inflammatory disease. Ophthal Plast Reconstr Surg. 2006;22:395-397.
- Orcutt JC, Garner A, Henk JM, Wright JE. Treatment of idiopathic inflammatory orbital pseudotumours by radiotherapy. Br J Ophthalmol. 1983;67(9): 570-574.
- Patel AK, Hoch S, Shindler KS. Mycophenolate mofetil treatment of steroid-resistant idiopathic sclerosing orbital inflammation. Clin Experiment Ophthalmol. 2011;39(9):912-913.
- Pemberton JD1, Fay A. Idiopathic sclerosing orbital inflammation: a review of demographics, clinical presentation, imaging, pathology, treatment, and outcome. Ophthal Plast Reconstr Surg. 2012;28(1):79-83.
- Porhajihosseini, S., Al-Sallami, A., & Al-Rubaye, S. (2022). Idiopathic lateral rectus myositis in preteens: A case report. Radiology Case Reports, 17(12), 4580–4583.
- Rosenbaum JT, Choi D, Harrington CA, et al: Gene expression profiling and heterogeneity of nonspecific orbital inflammation affecting the lacrimal gland. JAMA Ophthalmol. 2017; 135:1156.
- Rubin PA, Foster CS. Etiology and management of idiopathic orbital inflammation. Am J Ophthalmol. 2004;136:1041-1043.
- Schafranski MD. Idiopathic orbital inflammatory disease successfully treated with rituximab. Clin Rheumatol. 2009;28:225-226.
- Sergott R, Glaser J, Charyulu K. Radiotherapy for idiopathic inflammatory orbital pseudotumor: indications and results. Arch Ophthalmol. 1981;99(5):853-856.
- Sharma A, Foster RS, Suh DW, et al: Idiopathic enlargement of the extraocular muscles in young patients: A case series. Am J Ophthalmol. 2016; 161:206.
- Smith JR, Rosenbaum JT. A role for methotrexate in the management of non-infectious orbital inflammatory disease. Br J Ophthalmol. 2001;13:1220-1224.
- Spindle J, Tang SX, Davies B, et: Pediatric idiopathic orbital inflammation: clinical features of 30 cases. Ophthal Pl Reconstr Surg. 2016; 32:270.
- Suhler EB, Lim LL, Beardsley MD, et al: Rituximab therapy for refractory orbital inflammation: Results of a phase 1/2, dose-ranging, randomized clinical trial. JAMA Ophthalmol. 2014; 132:572.
- Verma S, Kroeker KI, Fedora RN. Adalimumab for orbital myositis in a patient with Crohn’s disease who discontinued infliximab: a case report and review of the literature. BMC Gastroenterol. 2013;13:59.
- Wallace ZS, Khosroshahi A, Jakobiec FA, et al. IgG4-related systemic disease as a cause of “idiopathic” orbital inflammation, including orbital myositis, and trigeminal nerve involvement. Surv Ophthalmol. 2012;57:26-33.
- Wilson MW, Shergy WJ, Haik BG. Infliximab in the treatment of recalcitrant idiopathic orbital inflammation. Ophthal Plast Reconstr Surg. 2004;20:381-383.
- Winn BJ, Rootman J. Sclerosing orbital inflammation and systemic disease. Ophthal Plast Reconstr Surg. 2012;28(2):107-118.
- Yuen SJ, Rubin PA. Idiopathic orbital inflammation: distribution, clinical features, and treatment outcome. Arch Ophthalmol. 2003;121(4):491-499.
- Zborowska B, Ghabrial R, Selva D, et al. Idiopathic orbital inflammation with extraorbital extension: case series and review. Eye. 2006;20:107-113
Financial disclosures
Reviewers
Sana Ali: No disclosures
