Myogenic Ptosis
Updated May 2025
Richard C. Allen, MD, PhD, FACS
Myogenic ptosis consists of any ptosis secondary to inherent levator or Muller muscle dysfunction. Using this definition, this could encompass many types of ptosis. However, as the pathophysiology of ptosis has been delineated, it becomes clear that many ptoses with signs of muscle dysfunction are actually due to upstream issues – congenital ptosis is now believed to be due to dysinnervation (Bosley, Curr Opin Ophthalmol 2013) and, obviously, even though a Horner syndrome and third nerve palsy might show evidence of abnormal muscle during surgery, they too are neurogenic. Myasthenia gravis is a disorder of the neuromuscular junction. This results in the causes of myogenic ptosis being considerably narrowed. Although involutional ptosis has traditionally been thought to be secondary to aponeurotic disinsertion or stretching, there are some who believe that there might be a myogenic component to it. That being said, involutional ptosis will not be the subject of this outline. Rather, the three most common causes of myogenic ptosis will be reviewed: chronic progressive external ophthalmoplegia (CPEO), myotonic dystrophy, and oculopharyngeal muscular dystrophy (OPMD). In addition, see related article on Frontalis Suspension and Alternative Procedures.
Establishing the diagnosis
Etiology
- CPEO, OPMD, and myotonic dystrophy are all due to genetic alterations (Allen, 2013).
- CPEO is caused by mutations in genes that are involved in mitochondrial function (Biousse and Newman, 2003). This has traditionally been described as mutations in the mitochondrial genome; however, it is also recognized that mutations in genes that are encoded in the nucleus and involved in mitochondrial function can cause CPEO. This results in there being three potential inheritance patterns: maternal, autosomal dominant, and autosomal recessive. CPEO caused by mtDNA mutations reveals rearrangement of segments of mtDNA in the form of deletions and duplications and, less commonly, point mutations. There are currently seven genes from the nuclear genome known, mutations in which cause CPEO. Autosomal dominant: ANT1, C10orf2, POLG, POLG2, RRM2B, and DNA2. Autosomal recessive: TYMP and POLG.
- Myotonic dystrophy is an autosomal dominant disease caused by mutations in the DMPK gene. The gene contains a noncoding trinucleotide repeat (CTG) that is expanded in affected individuals. Normal individuals have 5 to 37 repeats, while mildly affected individuals have 50-150 repeats, classically affected individuals have 100-1000 repeats, and congenital onset is associated with more than 2000 repeats (Harley et al., 1993). The repeat can lengthen as it is passed through the generations, a phenomenon known as anticipation.
- OPMD is an autosomal dominant disease caused by mutations in the PAPBN1 gene (Brais et al., 1998). The most common mutation identified is a small triplet repeat expansion. Due to the relative stability of this repeat and the fact that it is short, there is no significant correlation of disease severity with repeat length, and there is no anticipation.
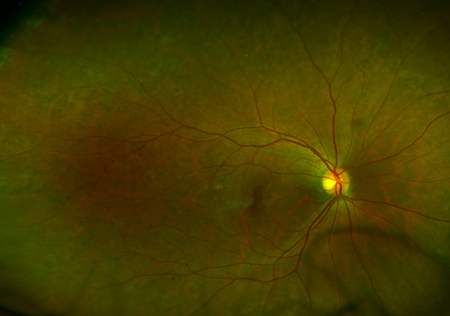
Figure 1. CPEO retinopathy. Courtesy Raymond Cho, MD.
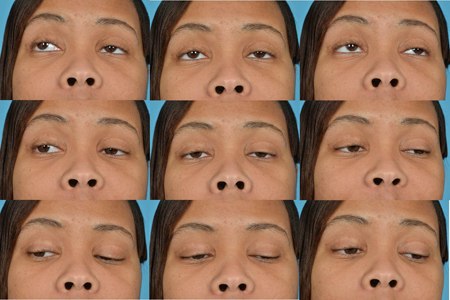
Figure 2. CPEO. Courtesy Raymond Cho, MD.
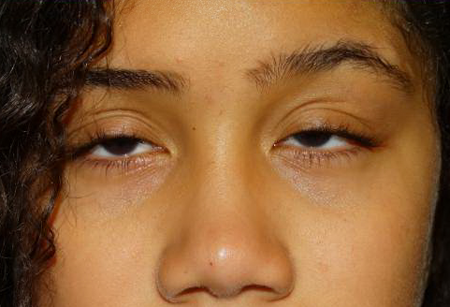
Figure 3. CPEO ptosis. Courtesy Anne Barmettler, MD.
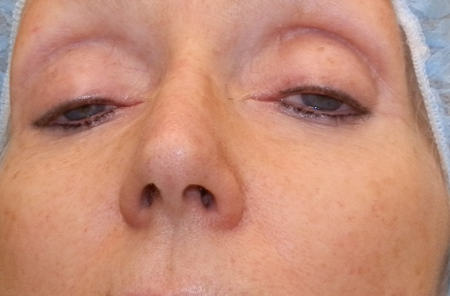
Figure 4. CPEO. Courtesy Brett Davies, MD.
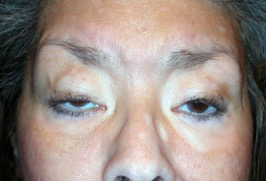
Figure 5. CPEO. Courtesy Michael J. Hawes, MD.
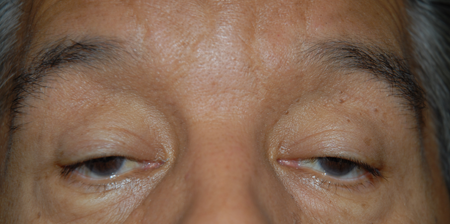
Figure 6. OPMD. Courtesy Richard C. Allen, MD, PhD, FACS.
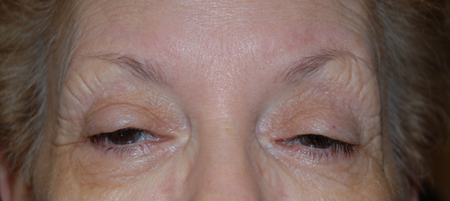
Figure 7. OPMD. Courtesy Richard C. Allen, MD, PhD, FACS.
Epidemiology
- Due to the genetic character of these diseases, there are no risk factors other than having parents who carry the mutations.
- In CPEO, there can be significant variability within families who carry mtDNA mutations due to the fact that in each cell, there are multiple mitochondria, and in each mitochondrion, there are multiple copies of the circular DNA. Due to this, within a family, there can be variability with regards to the proportion of mitochondria that carry a mutation and the proportion of circular DNA in each mitochondrion that have the mutation (Biousse and Newman, 2003).
- In OPMD, there are significant geographic clusters of patients in Quebec (French descent), Northern New Mexico (Hispanic descent), and Israel (Bukhara Jewish descent) (Becher et al., 2001) (Blumen et al., 2000).
Pertinent elements of history
Family history is an important piece of information to obtain in these patients. However, due to the variability of penetrance and expression in CPEO and myotonic dystrophy, the family history might not be informative. For OPMD, recognition of French Canadian or Hispanic New Mexican descent is useful.
Clinical features
For each of these diseases, ptosis should be noted with evidence of subnormal levator strength. The subnormal levator strength can sometimes be difficult to elicit early in the disease. Traditionally, levator function is used to determine levator strength; however, early in the disease levator function can still be “normal” (15 mm) (Frueh and Musch, 1996). Looking at the velocity of the lid from downgaze to upgaze can be helpful, noting a slower velocity in these patients even though LF is normal. The age of onset of CPEO and myotonic dystrophy is variable; however, earlier onset is associated with more severe ocular and systemic disease (Harley et al., 1993). The age of onset of OPMD is around the age of 50, with 99% of patients with the mutation having symptoms by the age of 70 (Brais et al., 1999).
- CPEO
- Reduced extraocular motility
- Reduced orbicularis strength
- Poor Bells phenomenon (related to reduced extraocular motility)
- Kearns-Sayre syndrome
- Retinal pigmentary changes
- Cardiac conduction disorders
- Cerebellar ataxia
- High CSF protein
- Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE)
- Mutations in TYMP
- Autosomal recessive
- Autosomal recessive cardiomyopathy ophthalmoplegia (ARCO)
- Mutations in POLG
- Autosomal recessive
- Myotonic dystrophy
- Reduced extraocular motility (not as much as CPEO)
- Reduced orbicularis strength (more than CPEO)
- Significant meibomian gland dysfunction
- Poor Bells phenomenon (related to reduced extraocular motility)
- Myotonia
- Cardiac conduction abnormalities
- Polychromatic (Christmas tree) cataracts
- Hypogonadism
- Frontal balding
- OPMD
- Reduced supraduction (not as much as CPEO or myotonic).
- Intact orbicularis strength
- Intact Bells phenomenon
- Dysphagia
- Can precede or follow ptosis symptoms
- Patients might describe choking on food.
- Patients take longer to finish meals.
- Aspiration pneumonia later in the disease
- Some patients will eventually have a feeding tube.
- Dysphonia
- Patients can almost be diagnosed by the sound of their voice.
- Proximal muscle weakness
- Later in the disease
- Not recognized in the textbooks
Testing and evaluation for establishing diagnosis
- CPEO
- Due to the multiple potential genes involved and multiple different potential mutations, genetic testing has not become standard for CPEO.
- Muscle biopsy remains the mainstay of diagnosis.
- Easily accessible muscles such as the vastus lateralis or deltoid are the most commonly biopsied muscles.
- Some have advocated orbicularis oculi biopsy during eyelid surgery (Roefs et al., 2012).
- Pathology
- Ragged-red fibers
- Partial cytochrome C oxidase deficiency
- MRI has shown the EOMs to be smaller (Carlow et al., 1998).
- Myotonic dystrophy
- Genetic testing is appropriate to evaluate the triplet repeat length in the DMPK gene.
- Neurology consultation
- EMG can be helpful in diagnosis to demonstrate myotonia.
- OPMD
- Genetic testing is appropriate to evaluate the triplet repeat length in the PABPN1 gene.
- Muscle biopsy is no longer appropriate.
- Pathology showed intranuclear inclusions (INIs).
Evaluation to determine staging or impairment level
- Clinical examination
- Margin reflex distance 1 (MRD1)
- Palpebral fissure height (PF)
- Levator function (LF)
- Lid crease
- Orbicularis strength
- Extraocular motility
- Photographs
- To document level of impairment
- Ptosis visual fields
- With lids untapped and taped
- To document level of impairment
Risk factors
These are genetic diseases, so there are no risk factors other than having parents who carry the mutation for the particular disease.
Differential diagnosis
These diseases have significant overlap and can be difficult to distinguish from each other; therefore, each is on the differential diagnosis for the other.
Myasthenia gravis
- Elicit more variation of symptoms
- Should be able to fatigue in clinic
- Less symmetrical compared to OPMD, myotonic, and CPEO
- No family history
- Tensilon test or ice test
- Auto-antibodies
- Single fiber EMG
Undiagnosed congenital ptosis
- Should not be significantly progressive
- Look at old photos.
Involutional ptosis
- High lid crease
- Normal levator function
- Some patients with involutional ptosis will have subnormal LF.
- No significant extraocular motility deficit
- Although older individuals will often have decrease supraduction.
Patient management: treatment and follow-up
Natural history
- CPEO
- Progressive ptosis
- Progressive extraocular motility weakness
- Progressive orbicularis weakness
- Myotonic dystrophy
- Progressive ptosis
- Progressive extraocular motility weakness
- Progressive orbicularis weakness
- OPMD
- Progressive ptosis
- Orbicularis strength and extraocular motility remain largely intact.
Medical therapy
- No medical therapy at this time to improve the ocular issues
Radiation
- Not applicable to these diseases
Surgery
- Due to progressively worsening levator function in these three diseases, frontalis suspension may be the optimal repair technique
- Frontalis suspension
- Primary silicone frontalis suspension is arguably superior to other surgical interventions because it can be more effective and longer lasting (Allen et al., 2009) (Allen et al., 2012).
- Silicone is elastic, allowing closure especially in the setting of orbicularis weakness (Carter et al., 1996).
- Silicone is adjustable for recurrent ptosis over time.
- Creation of a lid crease, and therefore, attaining an excellent cosmetic result by following these 5 principles (Allen et al., 2014)
- Conservative skin excision
- No preaponeurotic fat excision
- Fixation of the sling to the anterior surface of the tarsus
- Retroseptal placement of the sling (Patrinely and Anderson, 1986)
- Incorporation of the levator aponeurosis into the skin closure
- Levator advancement/resection
- Might not be as effective as frontalis suspension (Allen et al., 2009)
- Operating on a muscle that is progressively getting more weak
- Sentencing the patient to reoperation
- Blepharoplasty
- Advocated by some as an alternative surgery (Burnstine and Putterman, 1999)
- Might be less effective and have greater recurrence compared to frontalis sling and levator advancement/resection (Allen et al., 2009)
- Might result in less satisfying cosmetic result if the lid crease is not addressed (Allen et al. 2014)
- Posterior approach surgery
- MMCR
- No formal studies
- MMCR can function as an internal levator advancement, so it is unlikely to be as effective as frontalis suspension.
- Fasanella-Servat
- Excision of tarsus can exacerbate dry eye concerns, especially in CPEO and myotonic patients.
- Produces an unstable tarsus, which will make eventual reoperation with a frontalis sling more difficult
Other management considerations
- Ptosis crutches
- Metal appliances attached to the back of spectacles
- As glasses are placed on the face, the metal crutch pushes into the superior sulcus, thus elevating the eyelids as the glasses are worn.
- A last-ditch effort for some patients or those who do not want surgery or are poor surgical candidates
Common treatment responses, follow-up strategies
- Goal for all 3 diseases is to balance the correction of ptosis with the potential for dry eye.
- Follow-up after frontalis suspension
- 1 week
- Check for significant asymmetry or dryness.
- rare to adjust this early unless significant problem
- 1 month
- Check for significant asymmetry or dryness.
- Consider adjusting for any asymmetry, over-correction, under-correction.
- 4 months
- Check height and dryness.
- Consider adjusting for any asymmetry, over-correction, under-correction.
- Yearly
- Check height and dryness.
- Consider adjusting for any asymmetry, over-correction, under-correction.
Preventing and managing treatment complications
Over-correction and exposure keratopathy
- Lubrication
- Artificial tears during the day
- Ointment at night
- Warm compresses to address meibomian glands
- Moisture goggles at night
- Sling revision/loosening
- Lower lid elevation to decrease PF and keep MRD1 the same.
Under-correction/decrease in height with time
- Sling revision/tightening
Asymmetry
- Sling revision
Sling exposure/infection
- An exposed sling is a colonized foreign body.
- Exposed slings should be removed.
- Replacement can be performed 1 month later.
Sling breakage
- Rare
- More likely to occur during revision
- Due to the risk of sling breakage during revision, patients should be counselled of this risk prior to undergoing revision, especially for minor asymmetry.
Disease-related complications
CPEO
- Cardiac conduction issues (Pfeffer and Mezei, 2012)
- Early onset disease should be evaluated by a cardiologist every year.
- Late onset disease should be screened at 3–5 year intervals.
Myotonic dystrophy
- Cardiac conduction abnormalities
- All patients should be evaluated by a cardiologist.
OPMD
- Dysphagia
- All patients should be evaluated by a GI specialist or ENT familiar with the disease.
- Treatments include periodic dilations, botulinum toxin, and surgery (cricopharyngeal myotomy) (Youssof et al., 2014) (Gomez –Torres et al., 2012) (Manjaly et al. 2012).
Historical perspective
OPMD in Quebec has been traced to Zacharie Cloutier and Saincte Dupont, who immigrated to Canada from France in 1634.
References and additional resources
- Ahn J, Kim NJ, Choung HK, et al. Frontalis sling operation using silicone rod for the correction of ptosis in chronic progressive external ophthalmoplegia. Br J Ophthalmol 2008;92:1685-1688.
- Allen RC. Genetic disease affecting the eyelids: what should a clinician know? Curr Opin Ophthalmol 2013;24:463-477.
- Allen RC, Jaramillo J, Black R, et al. Clinical characterization and blepharoptosis surgery outcomes in Hispanic New Mexicans with oculopharyngeal muscular dystrophy. Ophthal Plast Reconstr Surg 2009;25:103-108.
- Allen RC, Zimmerman MB, Watterberg EA, et al. Primary bilateral silicone frontalis suspension for good levator function ptosis in oculopharyngeal muscular dystrophy. Br J Ophthalmol 2012;96:841-845.
- Allen RC, Hong ES, Zimmerman MB, Morrison LA, Nerad JA, Carter KD. Factors affecting eyelid crease formation before and after silicone frontalis suspension for adult-onset myogenic ptosis. Ophthal Plast Reconstr Surg. 2014 [Epub ahead of print].
- Becher MW, Morrison L, Davis LE, et al. Oculopharyngeal muscular dystrophy in Hispanic New Mexicans. JAMA 2001;286:2437-40.
- Bernardini FP, de Concilis C, Devoto MH. Frontalis suspension sling using a silicon rod in patients affected by myogenic blepharoptosis. Orbit 2002;21:195-8.
- Biousse V, Newman NJ. Neuro-ophthalmology of mitochondrial diseases. Curr Opin Neurol 2003;16:35-43.
- Blumen SC, Korczyn AD, Lavoie H, et al. Oculopharyngeal MD among Bukhara Jews is due to a founder (GCG)9 mutation in the PABP2 gene. Neurology 2000;55:1267-70.
- Bosley TM, Abu-Amero KK, Oystreck DT. Congenital cranial dysinnervation disorders: a concept in evolution. Curr Opin Ophthalmol 2013;24:398-406.
- Brais B, Bouchard JP, Xie YG, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystroph. Nat Genet 1009;19:164-7.
- Brais B, Rouleau GA, Bouchard JP, Fardeau M, Tome FM. Oculopharyngeal muscular dystrophy. Semin Neurol 1999;19:59-66.
- Burnstine MA, Putterman AM. Upper blepharoplasty: a novel approach to improving progressive myopathic blepharoptosis. Ophthalmology 1999;106:2098-100.
- Carlow TJ, Depper MH, Orrison WW Jr. MR of extraocular muscles in chronic progressive external ophthalmoplegia. AJNR Am J Neuroradiol 1998;19:95-9.
- Carter SR, Meecham WJ, Seiff SR. Silicone frontalis slings for the correction of blepharoptosis: indications and efficacy. Ophthalmology 1996;103:623-30.
- Frueh BR, Musch DC. Evaluation of levator muscle integrity in ptosis with levator force measurement. Ophthalmology 1996;103:244-50.
- Gomez-Torres A, Abrante Jimenez A, Rivas Infante E, et al. Cricopharyngeal myotomy in the treatment of oculopharyngeal muscular dystrophy. Acta Otorrinolaringol Esp 2012;63:465-9.
- Harley HG, Rundle SA, MacMillan JC, et al. Size of unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am J Hum Genet 1993;52:1164-1174.
- Lelli GJ Jr, Musch DC, Frueh BR, et al. Outcomes in silicone rod frontalis suspension surgery for high-risk noncongenital blepharoptosis. Ophthal Plast Reconstr Surg 2009;25:361-5.
- Manjaly JG, Vaughan-Shaw PG, Dale OT, et al. Cricopharyngeal dilation for the long-term treatment of dysphagia in oculopharyngeal muscular dystrophy. Dysphagia 2012;27:216-20.
- Molgat YM, Rodrigue D. Correction of blepharoptosis in oculopharyngeal muscular dystrophy: review of 91 cases. Can J Ophthalmol 1993;28:11-4.
- Park, R. B., Akella, S. S., & Aakalu, V. K. (2022). A review of surgical management of progressive myogenic ptosis. Orbit, 42(1), 11–24. https://doi.org/10.1080/01676830.2022.2122514
- Patrinely JR, Anderson RL. The septal pulley in frontalis suspension. Arch Ophthalmol 1986104:1707-10.
- Pfeffer G, Mezei MM. Cardiac screening investigations in adult-onset progressive external ophthalmoplegia patients. Muscle Nerve 2012; 46:593-596.
- Roefs AM, Waters PJ, Moore GR, Dolman PJ. Orbicularis oculi muscle biopsies for mitochondrial DNA analysis in suspected mitochondrial myopathy. Br J Ophthalmol 2012;26:1296-1299.
- Wong VA, Beckingsale PS, Oley CA, Sullivan TJ. Management of myogenic ptosis. Ophthalmology 2002;109:1023-1031.
- Youssof S, Schrader RM, Romero-Clark C, et al. Safety of botulinum toxin for dysphagia in oculopharyngeal muscular dystrophy. Muscle Nerve 2014;49:601-3.
Financial disclosures
Reviewers
Viraj Mehta – No disclosures
