Anophthalmos, Microphthalmos, and Congenital Socket Malformations
Updated July 2025
Overview
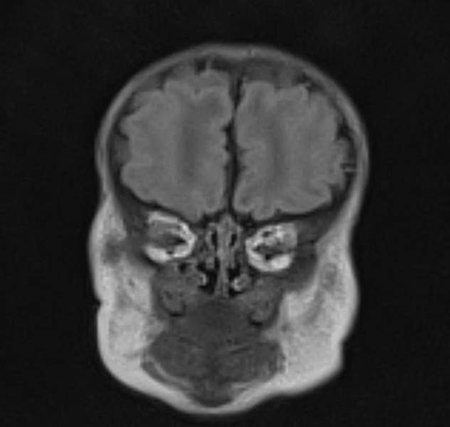
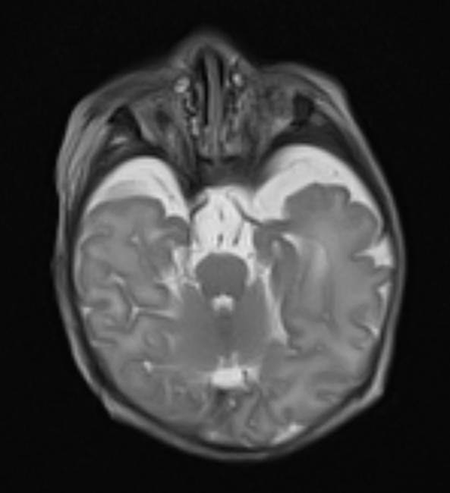
Anophthalmos represents growth failure in the primary optic vesicle, an emanation from the cerebral vesicle.
- It is actually a spectrum of disease. True anophthalmos is very rare. A small vestige of ocular tissue can generally be found.
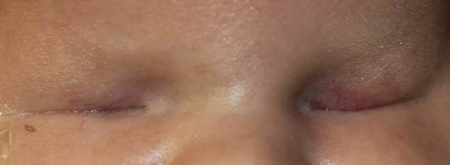
Microphthalmia and microphthalmos are synonymous nouns describing either the abnormally small eye or the process that results in an abnormally small globe.
- Microphthalmos with cyst – where the cyst is the dominant feature, lined with rudimentary sclera and neuroglia, communicating through a channel into the vitreous cavity.
- Most commonly a sporadic event, but important inheritance patterns exist with associated patterns of facial, neurologic and systemic abnormalities.
Epidemiology
In a study of 346,831 consecutive births in a geographically defined population in France including live births, still births, and terminations of pregnancy from 1979 to 2004, there were 87 infants with anophthalmia and microphthalmia for an incidence of 2.5 per 1,000 births (Birth Defects Research 2012; 94:147).
- Among the 87 infant with anophthalmia and microphthalmia, 90% had associated nonocular malformations.
The incidence in the US is estimated at 1.87 cases per 10,000 live births, with an estimated 780 new cases annually in the US (Archives of Ophthalmology 2011; 129:1077).
- This is much lower than the estimate in the French study.
In a study of live births, through the Surveillance of Eye Anomalies in the United Kingdom, 135 cases of anophthalmia and microphthalmia were identified over an 18-month period from 2006 to 2008, of which 55% had bilateral involvement, 78% had colobomas (43% had anterior and posterior coloboma, 43% only posterior coloboma, and 13% only anterior coloboma), and 60% (80 children) had extraocular abnormalities (Ophthalmology 2012; 119:362).
- Among the 80 children with extraocular abnormalities, craniofacial anomalies were most common. Developmental delay was present in 30 children (22%) and 16 children were hearing impaired (12%).
Genetics
Trisomy 13 and 18 are the most common chromosomal abnormalities among live births with anophthalmia and microphthalmia (European Journal of Medical Genetics, 2024).
The most common genetic abnormalities are deletions of 16p11.2, 16q11.2q12.2 and duplications of 10q24.31 and 15q11.2q13.1 (BMC Medical Genetics 2009; 10:137).
- Mutations in specific transcription factors critical to the development of the eye have been identified, particularly in consanguinous families with anophthalmos and microphthalmos.
- In a study of three consanguinous families with familial microphthalmos, homozygozity for mutations in the gene that encodes aldase dehydrogenase 1 was found (American Journal of Human Genetics 2013; 92:265).
- This enzyme is important in the formation of a retinoic acid gradient along the dorso-ventral axis during eye development.
- “Knockout” of this enzyme in mice causes malformations in the ocular and nasal regions – including ventral retinal shortening, lens rotation and persistence of primary vitreous.
- Homozygous mutation in SOX2 are common and can produce ocular disease as well as neurologic and pituitary abnormalities. SOX2 is an important transcription factor in neurologic development (BMC Medical Genetics. 12:172, 2011).
- GDF6 = growth and differentiation factor 6, part of a family of bone morphogenic proteins, important in neurologic development, and identified in anophthalmos and microphthalmos patients. Mutations in GDF6 have primarily been linked to Klippel-Feil syndrome which includes congenital malfusion of the cervical spine vertebrae, low posterior hairline and short neck with limited mobility.
Noero et al (Ophthalmic Genetics, 2025) studied 50 children with microphthalmos or anophthalmos looking specifically for pathogenic variants of SOX2 mutations and did not detect any variants in this cohort. They concluded that this is not a frequent cause despite experimental evidence that supports the association.
Okoye et al (American Journal of Medical Genetics, 2023) found SOX2 pathogenic variants in patients with normal eyes but systemic anomalies such as urogenital abnormalities, developmental delay, gastrointestinal anomalies, pituitary dysfunction, complex movement disorders, and other neurologic issues.
- Therefore, although SOX2 pathogenic variants are the most commonly known genetic cause of microphthalmia or anophthalmia they also cause a spectrum of developmental anomalies with normal ocular development.
In a Brazil study of 17 patients with orofacial clefts (OC) and microphthalmia or anophthalmia, chromosomal microarray analysis did not identify pathogenic variants but whole exome sequencing identified genetic abnormalities in six individuals. (European Journal of Human Genetics, 2024)
Basharat et al (Genes, 2023) similarly found that genomic sequencing targeting FOXE3, PXDN and VSX2 provided a genetic diagnosis in seven cases of microphthalmia or anophthalmia in consanguineous families.
- Therefore, gene sequencing seems to be the best means of identifiying genetic associations with anophthalmos and microphthalmos.
Syndromes
Amniotic band sequence, oculo-auriculo-vertebral spectrum, CHARGE syndrome and VACTERL association are the most recognizable non chromosomal associations (European Journal of Medical Genetics, 2024)..
Lenz microphthalmia syndrome is X-linked recessive, associated with cardiac (ventriculoseptal defect, patent ductus arteriosus) and skeletal anomalies (kyphoscoliosis, spinal stenosis) and mental retardation.
Microphthalmia linear skin defect syndrome is X-linked dominant, male lethal, associated with mutations in holocytochrome c-type synthase which is part of the mitochondrial respiratory chain.
Ma et al (Journal of American Association for Pediatric Ophthalmology & Strabismus, 2023) studied 168 patients with microphthalmia or anophthalmia (2017-2021) and found that 28 (16.7%) had fellow eye abnormalities, notably fundus abnormalities. The most common posterior segment finding was coloboma (7.7%), followed by optic nerve dysplasia (3.0%), and familial exudative vitreoretinopathy.
Varde (Orbit, 2025) described the management of lacrimal drain maldevelopment in arrhinia-microphthalmia syndrome with unilateral or bilateral absence of the nasal cavity and ocular anomalies including microphthalmia.
- Bosma arhinia microphthalmia syndrome (BAMS, OMIM #603457) is a rare autosomal dominant disorder caused by heterozygous variation in the SMCHD1 gene on chromosome 18p11.
- In addition to microphthalmia, and absence or hypoplasia of the nose, patients may have choanal atresia, palatal abnormalities, hypogonadism, and cryptorchidism.
- Stenz (Journal of Pediatric Ophthalmology & Strabismus, 2024) recommended dacryocystectomy to treat dacryocystocele in these patients.
Natural history
Potential for visual development is usually absent or very limited.
In most cases of microphthalmos and in all cases of anophthalmos, the abnormal eye does not enlarge and thereby fails to stimulate growth of associated adnexal tissues. The ipsilateral orbit and eyelids do not grow normally and become out of proportion to the contralateral side, leading to facial asymmetry and additional deformity.
Management
Goals
- Symmetric appearance of eyelids, orbit and face
- Ability to comfortably wear an ocular prosthesis
Chireh et al (ACTA Paediatrica, 2025) interviewed 15 patients with microphthalmia or anophthalmia, aged 15-31 years, specifically focusing on daily life challenges and found that most patients viewed their condition as a natural part of life.
Cyst and microphthalmos
Groot et al (Acta Opthalmologica, 2024) followed 74 patients with unilateral or bilateral microphthalmia (2013-2022) and found that over 80% of patients with unilateral microphthalmia managed with serial conformers achieved reasonable symmetry of orbital width and height. The emphasized the value of 3D-printed conformer therapy starting at age <1 year.
Groot et al (British Journal of Ophthalmology, 2023) also noted that horizontal palpebral fissure length increases for ten months after treatment with 3D-printed conformer therapy and increases only slightly beyond ten months.
Ma et al (Journal of Plastic, Reconstructive & Aesthetic Surgery, 2024) retrospectively studied161 patients with microphthalmia or anophthalmia treated with self-inflating hydrogel expansion and found good expansion of palpebral height and width.
- 15 patients experienced extrusion or migration of the expander.
In a direct comparison of orbits with microphthalmos and cyst, 17 orbits were treated with surgical removal of the cyst, to facilitate fitting of a conformer, and 17 orbits had conformers fit without removal of the cyst (six additional orbits were treated in other ways). A similarly good cosmetic result was obtained in both groups (BJO 87:860, 2003).
The cyst itself can act as a tissue expander. A large orbital cyst can be a powerful stimulator of tissue expansion, even if it precludes conformer use.
When the cyst is not removed and the patient is not wearing a conformer, a reasonable compromise is to remove the orbital cyst at age 5, at which point orbital volume is about 90% of an adult and cosmesis becomes more important as the child begins school.
Aspirating the cyst contents can assist in fitting a conformer temporarily though reaccumulation is anticipated.
Ethanolamine oleate sclerotherapy can promote cyst resolution, after aspiration of the cyst contents, when there is no visual potential in the microphthalmic eye (OPRS 23:307, 2007).
Orbital expansion
The orbit is not fully grown until puberty and any effort at tissue expansion until then can be helpful. However, some parents (and ocularists) might be satisfied with eyelid expansion alone to facilitate prosthesis wear, and might elect to forego orbital surgery on the child.
Inflatable balloon expanders
An inflatable balloon tissue expander can be inserted as a means of manual tissue expansion, but inflation of the balloon can cause severe pain. With inflation, the orbital pressure can reach 150–200 mm Hg. The normal adult human orbital pressure is 3–6 mm Hg. Therefore the slow steady pressure exerted by a hydrogel tissue expander is preferred by some, since it creates pressures of approximately 20–30 mm Hg.
A bicoronal flap and lateral orbitotomy are sometimes needed for insertion of a balloon expander, although a newer balloon device that is implanted anteriorly and fixated to the orbital rim has been described (Am J Ophthalmol 151:470, 2011).
Hydrogel expanders
Wiese introduced self-filling hydrogel expanders in 1999 (J Craniomaxillofacial Surgery 27:72, 1999). Hydrogel expanders are made of a highly hydrophilic compound consisting of N-vinylpyrrolidone and methyl methacrylate, also commonly used to make soft contact lenses and some intraocular lenses.
The expected tissue expansion with a hydrogel expander is modest. In a study of 17 microphthalmic orbits treated with hydrogel expanders, the orbital volume expanded to 74%–83% of the contralateral side (J Am Ass Ped Ophthalmol Strab 16:458, 2012).
Hydrogel spheres are available in sizes including 6, 8 and 9 mm in diameter. The swelling time in vitro in normal saline is 1–4 days and final diameters of 12, 15, 18, 20, and 22 respectively are expected. The orbital sphere implant is placed through a small lateral soft tissue incision. In vivo, the expansion occurs over several weeks. Maximum expansion is expected within 30 days and the expander can remain in place for several months.
It may be appropriate the leave the hydrogel implant in place for years but this risks potential complications including overexpansion. Once removed the implant can be replaced with another hydrogel implant, a conventional orbital implant or a dermis fat graft.
Tissue wrapping of hydrogel implant
This might help control the rate of hydrogel expansion. In an in vitro study, 5.0-ml orbital hydrogel expander implants were placed in beakers containing 0.9% sodium chloride solution, either unwrapped, or placed in porcine sclera wrapping, or porcine fascia lata wrapping (wrappings were 5.5 x 5.5 cm). Final volume of the fascia-wrapped implant was 3 ml compared with 5 ml for the sclera wrapped and control implants (OPRS 27:327, 2011).
As an alternative to orbital spheres, small expandable pellets can be inserted in the socket which are 8 mm in length, 2 mm in diameter and have an expected hydrated state of 0.24 ml in volume.
Dermis fat grafts
Autologous dermis fat grafts can be used to exert orbital pressure and expand the orbit and lids by taking advantage of natural growth of the graft. (JAAPOS 5:367, 2001). Late need for debulking of the graft due to excessive growth has been reported in children less than 4 years old at the time of graft placement (OPRS 14:81, 1998).
Harvest and placement of the dermis fat graft can be accomplished in the first few months of life, although many surgeons will initially initiate eyelid and fornix expansion until the child is 6–12 months old before considering orbital surgery.
Eyelid and fornix expansion
Serial placement of custom rigid conformers can expand the eyelid and fornices. Each conformer is larger than the previous one, enough to cause expansion but not so large that is causes pain or extrusion. Placement and exchange may require general anesthesia and may require a suture tarsorrhaphy.
Hemisphere hydrogel expanders are alternative options for expansion of the eyelid. Only 24–48 hours after placement of the hemisphere expander the fornices can be adequate to allow fitting of a first conformer.
The hemisphere implant is placed in the fornices, as a regular conformer, and held in place with fornix reconstruction sutures tied externally.
The hemispheres are available in sizes including 6, 8, 9 and 10 mm in diameter. The swelling time in vitro in normal saline is only 1–2 days and final diameters of 11, 14, 18, and 20 respectively are expected. Regular follow-up is needed, even on a weekly basis, to the ocularist and/or surgeon while the eyelids are being expanded.
Specific complications from treatment
A hydrogel implant can migrate as it expands, possibly extruding rather than expanding the tissues, and extending beyond the orbital rim, precluding conformer or prosthesis wear.
Historical perspective
The noted ocularist, Paul Gougelman, wrote in 1937 that “ophthalmologists are beginning to realize that it is possible to fit a prosthesis in cases of (microphthalmos and) congenital blindness of one eye for cosmetic improvement, many patients, varying in age from 3 months to early adult life, have been referred for such fitting…it has been my experience over a period of many years that in such cases the natural globe presents an ideal foundation for the introduction of a prosthesis.”
References
- Atique Tacla M; de Mello Copelli M; Pairet E; et al: Molecular investigation in individuals with orofacial clefts and microphthalmia-anophthalmia-coloboma spectrum. Eur J Hum Gen 2024; 32(10):1257-1266.
- Basharat R, Rodenburg K, Rodriquez-Hidalgo M, et al: Combined Single Gene Testing and Genome Sequencing as an Effective Diagnostic Approach for Anophthalmia and Microphthalmia Patients. Genes 2023; 14(8): )08 01.
- Beby F, Commeaux C, Bozon M, et al: New phenotype associated with an arg116cys mutation in the CRYAA gene: Nuclear cataract, iris coloboma and microphthalmia. Arch Ophthalmol 125:213, 2007.
- Cape CJ, Zaidman GW, Beck AD, Kaufman AH: Phenotypic variation in ophthalmic manifestations of MIDAS (microphthalmos, dermal aplasia, and sclerocornea). Arch Ophthalmol 122:1070, 2004.
- Chireh E, Nordquist J, Gronlund MA, Fahnehjelm KT: Healthcare, school and daily life experiences of patients with microphthalmia or anophthalmia and their parents. Acta Paediatr 2025; 114(3): 619-627.
- Gossman MD, Mohay J, Roberts DM: Expansion of the human microphthalmic orbit. Ophthalmology 106:2005, 1999.
- Gougelman P: Fitting of prostheses for patients with cryptophthalmos and extreme microphthalmos. Arch Ophthalmol 18:774, 1937.
- Groot ALW, Remmers JS, Lissenberg-Witte Bl, et al: Workflow and treatment results for computer-aided design and 3D-printed conformer therapy of congenital anophthalmia and microphthalmia. Br J Ophthalmol 2023; 107(9):1239-1245.
- Groot ALW, de Graaf P, Remmers JS, et al: Long term follow-up of axial length and orbital dimensions in congenital microphthalmia and anophthalmia. Acta Opthalmologica 2024; 102(6):e935-e945.
- Harding P, Moosajee M: The molecular basis of human anophthalmia and microphthalmia. J Dev Biol 2019; 7(3):16
- Kokitsu-Nakata NM, Segarra VCD, Tonello C, et al: https://orcid.org/0000-0003-1532.
- Li B: Research status of congenital microphthalmos with orbital cyst. Int Ophthalmol 2025; 45:13.
- Ma L, Li Y, Zhang H, et al: Abnormalities of the contralateral eye in unilateral congenital anophthalmic or blind microphthalmic patients. J Am Assoc Ped Ophthalmol Strab 2023; 27(1):34.e1-34.
- Ma L, Hou Z, Zhang J, et al: Stepwise self-inflating hydrogel expansion for congenital anophthalmia and blind microphthalmia: Over 15 years’ experience in China. J Plast Reconstr Aesth Surg 2024; 90:40-46.
- Mclean CJ, Ragge NK, Jones RB, Collin JRO: The management of orbital cysts associated with congenital microphthalmos. Br J Ophthalmol 87:860, 2003.
- Noero J, Weber M, Chassaing M, et al: Genetic screening of the RNA-binding protein RBM24 and its binding sites in the SOX2 3’ untranslated region in a cohort of 50 patients with microphthalmia. Ophthal Genet 2025; 46(3): 256-260.
- Okoye O, Capasso J, Kopinsky SM, et al: SOX2 pathogenic variants with normal eyes: Expanding the phenotypic spectrum. Am J Med Gen 2023; 191(8):2198-2203.
- Shah SP, Taylor AE, Sowden JC, Ragge NK, et al: Anophthalmos, microphthalmos, and coloboma in the United Kingdom: Clinical features, results of investigations, and early management. Ophthalmology. 119:362, 2012
- Skalicky SE, White AJR, Grigg JR, et al: Microphthalmia, anophthalmia, and coloboma and associated ocular and systemic features: Understanding the spectrum. Arch Ophthalmol 131:1517, 2013.
- Slavotinek AM: Eye development genes and known syndromes. Molecular Genetics & Metabolism. 104:448, 2011.
- Stenz EC, Nyalakonda RR, McCulley TJ, Chen Y: Dacryocystocele and subsequent dacryocystectomy in a patient with Bosma arhinia microphthalmia syndrome (BAMS): A case report and review of literature. J Ped Ophthalmol Strab 2024; 61(2):e16-e18.
- Stoll C, Dott B, Alembik Y, et al: Associated anomalies in anophthalmia and microphthalmia. Eur J Med Gen 2024; 67:104892.
- Varde MA, Ali MJ: Dilemmas in the management of lacrimal drainage anomalies in BOSMA (congenital arrhinia-microphthalmia) syndrome. Orbit 2025; 44(1): 121-124.
- Weiss AH, Kousseff BG, Ross EA, Longbottom J: Simple microphthalmos. Arch Ophthalmol 107:1625, 1989.
- Weiss AH, Kousseff BG, Ross EA, Longbottom J: Complex microphthalmos. Arch Ophthalmol 107:1619, 1989
- Zapata S, Durairaj VD: Bilateral microphthalmia with orbital cysts in Wolf-Hirschhorn syndrome. Arch Ophthalmol 126:876, 2008.
Additional resources
Microphthalmia, Anophthalmia, and Coloboma Genetic Epidemiology in Children (MAGIC) – ClinicalTrials.gov ID NCT06293560
- Sponsor Baylor College of Medicine
- https://clinicaltrials.gov/study/NCT06293560?cond=Microphthalmia&rank=2
Pathogenesis and Genetics of Microphthalmia, Anophthalmia and Uveal Coloboma (MAC) – ClinicalTrials.gov ID NCT01778543
- Sponsor National Eye Institute (NEI)
- https://clinicaltrials.gov/study/NCT01778543?cond=Microphthalmia&rank=3
Eye Devices for Anophthalmia and Microphthalmia
EyeWiki: Microphthalmos
Financial disclosures
Reviewers
Simeon Lauer: No disclosures
