Basal Cell Nevus Syndrome (Gorlin-Goltz Syndrome)
Updated August 2024
Matthew C. Sniegowski, MD; Edward J. Wladis, MD
Basal cell nevus syndrome (BCNS; OMIM 109400) was first described as a distinct syndrome with the triad of multiple basal cell carcinomas (BCCs), jaw keratocysts and skeletal abnormalities by Gorlin and Goltz in 1960. It is a rare autosomal dominant disorder in which patients develop multiple BCCs at a young age as well as the constellation of findings of palmar pits, acquired odontogenic keratocysts, ectopic calcification of the falx cerebri and hypertelorism. Males and females are equally affected. All races are affected, although Caucasians are most commonly affected.
Establishing the diagnosis
BCNS is an autosomal dominant multisystem disease with complete penetrance and variable expression. The clinical diagnosis of BCNS is based on 2 major criteria, 1 major and 2 minor criteria or 1 major criteria and molecular genetic confirmation (Zhu, JAMA Dermatol 2014) (Bree, Am J Med Genet 2011)
Major criteria include
- BCC prior to 20 years old or excessive numbers of BCCs out of proportion to prior sun exposure and skin type
- Odontogenic keratocyst of the jaw prior to 20 years of age (Figure 1)
- Palmar or plantar pitting (Figures 2 and 3)
- Calcification of the falx cerebri
- Medulloblastoma (typically desmoplastic)
- First degree relative with BCNS
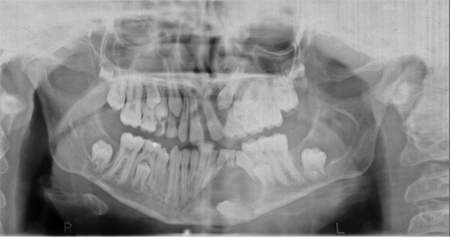
Figure 1. Odontogenic cysts. Courtesy Richard C. Allen, MD, PhD, FACS.
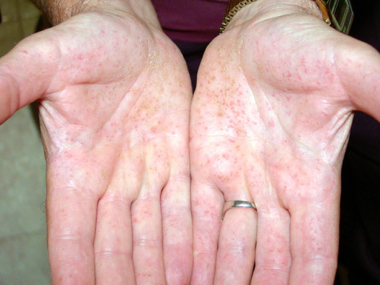
Figure 2. Palmar pits. Courtesy Michael J. Hawes, MD.
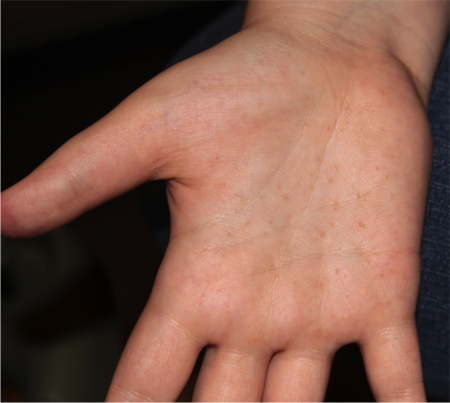
Figure 3. Palmar pits. Courtesy Richard C. Allen, MD, PhD, FACS.
Minor criteria include
- Rib anomalies
- Vertebral anomalies, kyphoscoliosis, polydactyly or other specific skeletal malformations
- Macrocephaly
- Cleft/lip palate
- Ovarian/cardiac fibroma
- Lympho-mesenteric cysts
- Hypertelorism, strabismus, congenital cataracts or congenital glaucoma
In most instances BCNS is a clinical diagnosis, for this reason genetic testing is not a diagnostic requirement. A recent consensus group recommended genetic testing only in the following situations:
- Prenatally if there was a known familial mutation
- To confirm the diagnosis in patients with some signs but not meeting the diagnostic criteria of BCNS
- In patients with a positive family history but without clinical signs of basal cell nevus syndrome (Tang NEJM 2012). Additionally, tarsal cysts may be present. (Wolkow, 2018)
Etiology
- BCNS is an autosomal dominant disease linked to chromosome 9q22.3-q31 (PTCH1 gene)
- Approximately 20-40% of cases are thought to be the result of spontaneous PTCH1 mutations
- Germ-line mutation results from gene rearrangements in approximately 70% of cases
- Results in truncation of the coded protein (Lo Muzio, Orphanet J Rare Dis 2008).
- Protein patched homolog 1(PTCH1) gene produces a sonic hedgehog receptor protein (PTCH1) which is a transmembrane receptor. This receptor normally functions as a tumor suppressor, inhibiting the downstream receptor Smoothened (Smo). When the PTCH1 gene is mutated (as in BCNS) or secreted Hedgehog protein to binds to PTCH1, this results in Smo no longer being inhibited and subsequent activation of the Hedgehog pathway. When Smo is no longer repressed by PTCH1, glioma-associated oncogene homolog (GLI) gene is activated with consequent cellular proliferation and tumorigenesis.
- Spontaneous BCCs most often arise from a sporadic loss of function mutation in the PTCH1 gene, whereas, patients with BCNS have a germ-line mutation in PTCH1 leading to the development of multiple BCNS (Kiwilsza, Med Sci Monit 2012) (Athar, Cancer Res 2014).
- Based on Knudson’s “two hit hypothesis.” like retinoblastoma, patients with basal cell nevus syndrome have inherited a germ-line mutation in the PTCH1 gene, however, they still require a second hit (environmental UV exposure/radiation/etc) prior to the development of basal cell carcinomas (Lam, Dermatol Surg 2013).
Epidemiology
- BCNS has been traced back to the period of the Egyptian Dynasties based on characteristic skeletal finding in mummies
- Estimated birth incidence of 1 in 19 000 to 1 in 164 000
- Greater than 90% of patients with BCNS will have a mutation of the PTCH1 tumor suppressor gene.
- Mutations in PTCH2 and suppressor of fused (SUFU) genes have also been identified as causative of BCNS in a minority of cases.
- BCNS unique in that it leads to both developmental defects as well as a predisposition to cancer (Gorlin, NEJM 1960) (Larsen, Dan Med J 2014)
History
- Family history
- Multiple BCCs at an early age
- Odontogenic keratocysts
Clinical features
- Appearance of BCCs at a young age
- Most of the BCCs will develop between puberty and age 35
- BCCs have been diagnosed as early as 2 years of age; no genotype/phenotype correlations have been identified.
- BCCs frequently occur in sun-exposed areas (face, back, chest), consistent with the two-hit hypothesis.
- BCCs can range in number from a few to several thousands.
- Milia, commonly in the periocular area
- Occurs in approximately 30% of patients
- Odontogenic keratocysts of the jaw
- Occur in up to 90% of patients
- More common in the mandible and the maxilla
- Palmar or plantar pits
- Occur in upwards of 85% of patients older than 20 years of age
- Small (2-3mm in diameter and 1-3 mm deep) lesions
- Increase with age
- Appearance is most visible if patients are asked to hold their hands under warm water for 10 minutes
- Pits have a red base in Caucasians and darker black base in pigmented patients
- Pathologically the pits are characterized by:
- Well-circumscribed hypokeratosis
- Basal cell hyperplasia
- Palisading basal keratinocytes
- Variable hypogranulosis and parakeratosis (Lam, Dermatol Surg 2013).
- Ectopic calcification of the falx cerebri
- Craniofacial abnormalities (Figure 4)
- Increased occipital-frontal head circumferences
- Frontal and biparietal bossing
- Mild hypertelorism
- Mandibular prognathia (Larsen, Dan Med J 2014).
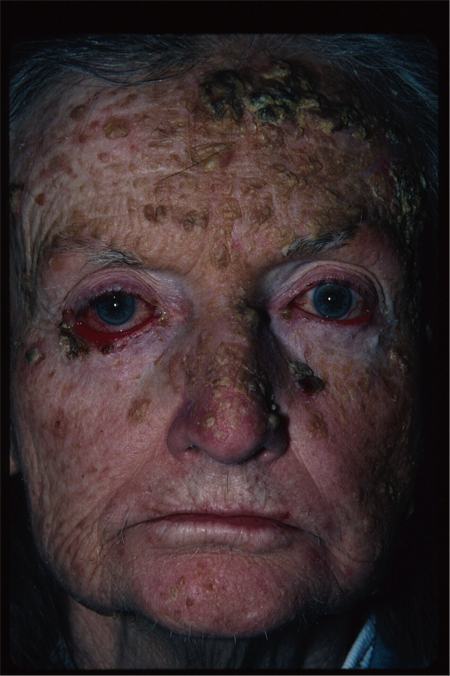
Figure 4. Basal cell nevus syndrome. Courtesy Rona Z. Silkiss, MD, FACS.
- Patients with BCNS tend to be much taller than their unaffected family members.
- Multiple nevi
- Seen in up to 70% of patients with BCNS who are older than 20 years of age
- Flesh colored, reddish-brown or pearly
- May resemble skin tags, moles or hemangiomas
- Can grow rapidly for a short time, most remain stable in size (Lo Muzio, Orphanet J Rare Dis 2008).
- Medulloblastoma
- Occur at an early age (less than 5 years of age)
- Thought to arise in approximately 2% of patients with BCNS
- More common in males than females
- Often of the desmoplastic variety
- Better prognosis than sporadically occurring medulloblastomas
- Ovarian cysts or fibromas
- Seen in 25 to 50% of females with BCNS
- Do not affect fertility
- Can undergo spontaneous torsion (Lo Muzio, Orphanet J Rare Dis 2008).
- Epidermoid conjunctival cysts
- Rare reports of these cysts causing irritation and requiring excision (De Craene, Orbit 2014).
- Cardiac fibromas
- Approximately 3-5% of all patients who have a cardiac fibroma will have BCNS
- Most commonly located in the left anterior ventricle wall
- May affect blood flow or lead to conduction defects
- Depending on size and involvement of the intraventricular septum (Lam, Dermatol Surg 2013).
Testing
- Patients with a positive family history of BCNS should be evaluated by their pediatrician
- Evaluation needs to assess for
- Increased head circumference
- Frontal or temporal bossing
- Cleft palate
- Bifid ribs
- Diagnosis in early childhood is challenging due to the fact that the various signs and symptoms gradually develop over time in BCNS
- When BCNS is suspected, patient need a through skin examination no later than the onset of puberty and a formal dental examination by late childhood.
- Skeletal and neuroimaging can be helpful in aiding in the diagnosis, depending on patient’s symptoms.
- Most patients with BCNS are diagnosed between ages 17 to 35, and develop their first BCC at a mean age of 23 years (Leger, Dermatol Online J 2011).
Testing for staging, fundamental impairment
- Patients diagnosed in early childhood with BCNS are recommended to:
- Undergo neurological examination every 6 months until age 3
- Then annually until age 7
- Evaluation is to detect a neurological deficit related to medulloblastoma
- From age 8 onward patients are recommended to undergo dental evaluation looking for odontogenic keratocysts
- Dermatologic evaluation should begin annually no later than the start of puberty
- Complete skin checks are recommended at a minimum of every 4-6 months depending on the appearance of new lesions
- Diagnostic studies such as MRI brain, echocardiography, abdominal ultrasound can all aid in the diagnosis as well as molecular genetic linkage studies (Lo Muzio, Orphanet J Rare Dis 2008)
Risk factors
Patients are exceptionally sensitive to actinic damage:
- Strong correlation between sun exposure and development of BCCs in BCNS patients.
- Use of sunscreens and avoidance of prolonged sun exposure can help to reduce the number of basal cell carcinomas that develop.
Differential diagnosis
- Bazex-Dupré-Christol syndrome (OMIM 301845)
- X-linked dominant disease of the hair follicle with follicular atrophoderma (breakdown of follicles on the skin), multiple BCCs, reduced body and scalp hair and localized anhidrosis
- Rombo syndrome (OMIM 180730)
- Autosomal-dominant syndrome characterized by BCCs, atrophoderma vermiculata (erythematous follicular papules on the cheeks which slowly progresses to atrophy with a reticular or honey-comb appearance), multiple milia, telangetasias, and a cyanotic redness
- Xeroderma pigmentosa (multiple types)
- Autosomal recessive defect in DNA mismatch repair, leading to development of BCC, SCC and melanoma
- Muir-Torre syndrome (OMIM 158320)
- Autosomal dominant disorder with sebaceous gland tumors (adenomas, epithelioma or carcinoma) and visceral malignancies (usually GI or GU)
- Trichoepithelioma papulosum multiplex (OMIM 601606)
- Autosomal dominant disorder with development of multiple trichoepitheliomas
Patient management: treatment and follow-up
Natural history
- Development of multiple, sometimes thousands, of BCCs beginning early in life
- Over the patient’s lifetime the multitude of BCCs requiring surgical excision can eventually lead to significant cosmetic deformity and morbidity.
- Patients with BCNS commonly have depressive symptoms.
- Life expectancy is not significantly different from patients without BCNS.
- Premature death occurs in less than 10% of patients with BCNS.
- If early death does occur it is usually due to medulloblastoma or metastatic BCC.
Medical therapy
- Treatment options for basal cell carcinomas are challenging in BCNS due to the sheer number of BCCs which develop.
- Moh’s micrographic surgery, 5% imiquimod, PDT or even vismodegib are all primarily based on sporadic BCCs. The therapy of patients with basal cell nevus syndrome must be individualized to the patient (Lam, Dermatol Surg 2013).
- Multidisciplinary management of patients is critical owing to the multitude of organ systems that may be involved.
- Topical 5% imiquimod, tretinoin and 5-fluorouracil have all been utilized for BCCs in basal cell nevus syndrome patients with varying success, these are most effective for superficial basal cell carcinomas (Lam, Dermatol Surg 2013).
- Oral high dose isotretinoin has been advocated by some to treat small (<1 cm lesions) and prevent new lesions, however a previous study assessing this found that only 8% of patients had complete clinical and pathological regression of their BCCs while all patients experienced moderate to severe toxicity (Bree, Am J Med Genet 2011).
- Hedgehog pathway inhibitors (e.g., Vismodegib (Erivedge); Genentech, South San Francisco, CA, USA; GDC-0449)
- Selective inhibitors of the Hedgehog pathway which bind to Smo and prevent downstream activation of the Hedgehog pathway
- Vismodegib gained FDA approval in January 2012 for metastatic or locally advanced and unresectable BCC. Sonidegib was approved in 2015.
- A recent prospective study assessed the utility of vismodegib in patients with BCNS. In this study 42 patients with BCNS were randomized in a 2:1 fashion to vismodegib or placebo. The study found that the treatment group had a statistically significant reduction in the number of new surgically eligible BCCs which developed, and this treatment effect was seen as early as one month into therapy. Vismodegib was also found to reduce the size of pre-existing surgically eligible BCCs and interestingly the palmar and plantar pits also disappeared during therapy. This study was ended early due to the significant response in the treatment arm. However, it is important to note that 54% of patients discontinued therapy early due to side effects, the most common side effects were dysgeusia, muscle cramps, hair loss and weight loss. The dysgeusia and muscle cramps typically resolved within one month of discontinuing therapy. Only one patient was able to complete the full 18 month course of therapy (Tang, NEJM 2012).
- Vismodegib appears to be very effective in addressing locally advanced adnexal and periorbital disease, with response rates of up to 100%. Similarly, many patients were able to avoid exenteration and maintain vision.
- One of the major considerations of Vismodegib therapy is whether the tumor suppressive effects can be maintained after discontinuation of the drug and to what extent; this is an area of ongoing research (Cowey, Dermatol Ther 2013).
- A study of Sonidegib evaluating the original BOLT trial, after which the drug was approved in 2015, as well as a 30-month update showed a disease control rate of 90.8%, and a median duration of progression-free survival of 22 months (Chen, L., Aria, A. B., Silapunt, S., Lee, H. H., & Migden, M. R. (2017). Treatment of Advanced Basal Cell Carcinoma with Sonidegib).
- Tazarotene (topical retinoid)
- Based on its efficacy in treating BCCs in PTCH1 knock out mice, there was interest in the use of tazarotenein prevention of BCC in patients with BCNS.
- A recent phase 2 clinical trial of topical tazarotene in patients with BCNS unfortunately found only a 6% response rate and the authors concluded that there was no evidence of either a chemo-preventative nor chemotherapeutic effect of tazarotene in patients with BCNS (Tang, Cancer Prev Res 2014).
Radiation
Radiotherapy must be used with extreme caution in patients with basal cell nevus syndrome owing to their propensity to form additional basal cell carcinomas at the irradiated site, sometimes many years after the radiation therapy.
- It has been recently established that patients with BCNS have a decreased expression of aldehyde dehydrogenase 1A1, which is thought to lead to their increased incidence of radiation induced tumors.5 Although radiotherapy is often used to treat medulloblastoma in patients with BCNS, the use of radiotherapy to treat cutaneous BCCs is typically avoided (Lam, Dermatol Surg 2013).
Surgery
Surgical excision with clear pathological margins of at least 4 mm is the gold standard of therapy for BCCs amenable to resection.
Moh’s micrographic surgery can be beneficial for BCCs on the face in particular where preservation of normal tissue is of utmost importance.
The challenge in patients with BCNS is that they have the potential to develop hundreds of basal cell carcinomas over their lifetime, thus making repeated surgical excision necessary and leading to significant morbidity (Lam, Dermatol Surg 2013).
Other management considerations
Photodynamic therapy (PDT) has been reported in several case reports in the literature to be a potential option for sustained prevention and treatment of patients with BCNS, since its mechanism of action is through oxidative damage rather than ionizing radiation.
- Some clinicians advocate that it should be the first-line therapy for individuals with basal cell nevus syndrome and multiple lesions.
- Patients are typically treated with PDT alone for lesions less then 2 mm thick and systemic photosensitizers are added for lesions thicker than 2 mm.
- Clearance rates for BCCs have ranged from 56-100% depending on the series.
- There is some evidence that PDT may also have a chemopreventative role, with several small case series showing a decreased incidence of new lesions following PDT.
- Drawback to the use of PDT is that the treatment sessions can be very painful (Schweiger J Drugs Dermatol 2010).
Carbon dioxide (CO2) and pulsed dye laser (PDL) have been used in several case reports.
- Success of these treatments is variable.
- Higher rates of success with more superficial BCCs.
- A single case report has demonstrated success with a 755 nm Alexandrite laser in a patient with massive tumor burden. The Alexandrite laser, due to its longer wavelength, is able to penetrate the entire dermis and in this single case report the authors found an 83% clearance rate (clinical and histological) at 7 months. This has been suggested as a treatment modality that warrants further study (Lam, Dermatol Surg 2013; Ibrahimi, Lasers Surg Med 2011).
Common treatment responses, follow-up strategies
Patients with BCNS have been shown to have a favorable response to a variety of treatment modalities.
The challenge with BCNS is that although the cure rates for an individual BCC lesion is similar to that of sporadically occurring BCCs, the patient with BCNS is at a much higher risk of developing subsequent BCCs owing to the germ-line PTCH-1 mutation.
Preventing and managing treatment complications
- Surgical excision is associated with scarring and potential loss of function in the periocular region.
- Small margins and use of Moh’s surgery can assist with normal tissue preservation.
- Radiotherapy will likely lead to a significant increase in the number of BCCs that form; these radiation-induced BCCs tend to be very aggressive.
Disease-related complications
- Multiple BCCs develop throughout life.
- Medulloblastoma may develop in early childhood.
- Prospective parents should be offered genetic counseling, given 50% risk of passing on the syndrome to a child.
Historical perspective
- Described as a distinct disease entity by Gorlin and Goltz in 1960
- Vismodegib was approved by the FDA for locally advanced and metastatic BCCs in 2012.
- Sonidegib was approved in July 2015 by the FDA for the treatment of locally advanced or metastatic basal cell carcinoma.
References and additional resources
- gorlinsyndrome.org
- bccns.org
- Athar M, Li C, Kim AL, et al. Sonic Hedgehog Signaling in Basal Cell Nevus Syndrome. Cancer Res. 2014 Sep 15;74(18):4967-75.
- Ben Ishai M, Tiosano A, Fenig E, et al. Outcomes of Vismodegib for periocular locally advanced basal cell carcinoma from an open-label trial. JAMA Ophthalmol. 2020;138:749-755.
- Bilir Y, Gokce E, Ozturk B, et al. Metastatic Basal cell carcinoma accompanying gorlin syndrome. Case Rep Oncol Med. 2014.
- Bree AF, Shah MR. Consensus Statement From the First International Colloquium on Basal Cell Nevus Syndrome (BCNS). Am J Med Genet. Part A 2011;155:2091–2097.
- Cowey CL. Targeted Therapy for Advanced Basal-Cell Carcinoma: Vismodegib and Beyond. Dermatol Ther (Heidelb) (2013) 3:17–31.
- Curragh DS, Huilgol SC, Selva D. Neoadjuvant Vismodegib in the management of locally advanced periocular basal cell carcinoma. Eye (Lond). 2021;35:2740-2745.
- De Craene S, Batteauw A, Van Lint M, et al. Subconjunctival epidermoid cysts in Gorlin-Goltz syndrome. Orbit. 2014 Aug;33(4):280-2.
- Demirci H, Worden F, Nelson CC, et al. Efficacy of Vismodegib (erivedge) for basal cell carcinoma involving the orbit and periocular area. Ophthalmic Plast Reconstr Surg. 2015;31:463-466.
- Eiger-Moscovich M, Reich E, Tauber G, et al. Efficacy of Vismodegib for the treatment of orbital and advanced periocular basal cell carcinoma. Am J Ophthalmol. 2019;207:62-70.
- Goldberg LH, Firoz BF, Weiss GJ, et al. Basal cell nevus syndrome: a brave new world. Arch Dermatol. 2010 Jan;146(1):17-9.
- Gonzalez AR, Etchichury D, Gil ME, Del Aguila R. Neoadjuvant Vismodegib and mohs micrographic surgery for locally advanced periocular basal cell carcinoma. Ophthalmic Plast Reconstr Surg. 2019;35:56-61.
- Gorlin RJ, Goltz RW. Multiple naevoid basal cell epithelioma, jaw cysts and bifid rib syndrome. N Engl J Med. 1960;262:908-12.
- Honavar SG, Shields JA, Shields CL, et al. Basal cell carcinoma of the eyelid associated with Gorlin-Goltz syndrome. Ophthalmology. 2001 Jun;108(6):1115-23.
- Ibrahimi OA, Sakamoto FH, Tannous Z, Anderson RR. 755 nm alexandrite laser for the reduction of tumor burden in basal cell Nevus syndrome. Lasers Surg Med. 2011 Feb;43(2):68-71.
- Kahana A, Unsworth SP, Andrews CA, et al. Vismodegib for preservation of visual function in patients with advanced periocular basal cell carcinoma: The visorb trial. Oncologist. 2021;26:e1240-e1249.)
- Kiwilsza M, Sporniak-Tutak K, Gorlin-Goltz syndrome – a medical condition requiring a multidisciplinary approach, Med Sci Monit, 2012; 18(9): RA145-153.
- Lam C, Ou JC, Billingsley EM. PTCH-ing It Together: A Basal Cell Nevus Syndrome Review. Dermatol Surg. 2013;39:1557–1572.
- Leger M, Quintana A, Tzu J, et al. Nevoid basal cell carcinoma syndrome. Dermatol Online J. 2011 Oct 15;17(10):23.
- Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Orphanet J Rare Dis. 2008 Nov 25;3:32.
- Larsen AK, Mikkelsen DB, Hertz JM, Bygum A. Manifestations of Gorlin-Goltz syndrome. Dan Med J. 2014 May;61(5):A4829.
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: An international multicentre case series. Eye (Lond). 2020;34:2076-2081.
- Ozgur OK, Yin V, Chou E, et al. Hedgehog pathway inhibition for locally advanced periocular basal cell carcinoma and basal cell nevus syndrome. Am J Ophthalmol. 2015;160:220-227 e222.
- Peck GL, DiGiovanna JJ, Sarnoff DS, et al. Treatment and prevention of basal cell carcinoma with oral isotretinoin. J Am Acad Dermatol. 1988, 19:176-185.
- Sagiv O, Ding S, Ferrarotto R, et al. Impact of food and drug administration approval of Vismodegib on prevalence of orbital exenteration as a necessary surgical treatment for locally advanced periocular basal cell carcinoma. Ophthalmic Plast Reconstr Surg. 2019;35:350-353.
- Sagiv O, Nagarajan P, Ferrarotto R, et al. Ocular preservation with neoadjuvant Vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol. 2019;103:775-780.
- Schweiger ES, Kwasniak L, Tonkovic-Capin V. A patient with neviod basal cell carcinoma syndrome treated successfully with photodynamic therapy: case report and review of the literature. J Drugs Dermatol. 2010 Feb;9(2):167-8.
- Tang JY, Chiou AS, Mackay-Wiggan JM. Tazarotene: randomized, double-blind, vehicle-controlled, and open-label concurrent trials for basal cell carcinoma prevention and therapy in patients with basal cell nevus syndrome. Cancer Prev Res (Phila). 2014 Mar;7(3):292-9.
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012 Jun 7;366(23):2180-8.
- Wolkow N, Jakobiec FA, and Yoon MK. Surv Ophthalmol, 63: 711-718, 2018.
- Wong KY, Fife K, Lear JT, et al. Vismodegib for locally advanced periocular and orbital basal cell carcinoma: A review of 15 consecutive cases. Plast Reconstr Surg Glob Open. 2017;5:e1424.
- Wright AT, Magnaldo T, Sontag RL, et al. Deficient expression of aldehyde dehydrogenase 1A1 is consistent with increased sensitivity of Gorlin syndrome patients to radiation carcinogenesis. Mol Carcinog. 2015 Jun;54(6):473-84.
- Zhu GA, Li AS, Chang AL. Patient with Gorlin syndrome and metastatic basal cell carcinoma refractory to smoothened inhibitors. JAMA Dermatol. 2014 Aug;150(8):877-9.
