Conjunctival Melanoma
Updated July 2025
Navdeep Nijhawan, MD; Ahsen Hussain, MD
This outline reviews conjunctival melanoma with emphasis on diagnosis and treatment, discussing differences in management. The emphasis is on conjunctival pigmented neoplasia as it relates to cutaneous and extraocular mucosal sites. For other conjunctival malignancies, see our companion outline.
Establishing the diagnosis
Etiology
- Tumors arise from melanocytes located among the basal cells of the conjunctival epithelium (Wong, Nanji et al. 2014)
- Melanocytes arise from the neural crest (Yanoff,. Sasani. Ocular Pathology 6th ed).
- Mucosal melanocytes do not have the same UV protection role as cutaneous melanocytes.
- Mucosal melanocytes possess immune processing functions including phagocytosis and antigen presentation.
- Cutaneous melanomas have been found to prominently carry oncogenic mutations in BRAF (serine/threonine kinase). These mutations have been found, but less commonly, in mucosal melanomas including conjunctival melanomas (Mihajilovic, Vlajkovic 2012).
- The T1799A BRAF mutation was identified in two of five (40%) conjunctival melanomas in one study (Goldenberg-Cohen, Cohen 2005).
- BRAF mutations were found in 3 of 15 conjunctival melanomas in another study (Spendlove, Damato 2004).
- In a larger study of 22 patients, The T1799A BRAF mutation was detected by sequencing in 5 of 22 tumors and this mutation tended to be associated with larger tumor diameter and greater depth of invasion (Gear, Williams 2004).
- Using PCR amplification, a study in Copenhagen found BRAF mutations in 39 of 111 conjunctival melanomas (Larson, AC, Dahl, C. 2016).
- Uveal melanomas frequently carry somatic mutations in Codon 209 of the GNAQ gene as the first initiating events, evident in 30-50% of cases, but these are not found in conjunctival melanomas (Dravitman-Storobinsky, Cohen 2010).
- Conjunctival melanomas harbored overaction of the oncogenic mTOR pathway, a target of rapamycin, in a study of eight tumors (Populo, Soares 2010).
Epidemiology
- Incidence of conjunctival melanoma 0.45–0.8/million (Shildkrot, Wilson 2010)
- There is a trend of increasing frequency among white men in the US (Shildkrot, Wilson 2010).
- Male:Female ratio is about equal.
|
Study |
Female/Male Ratio |
|
Damato et al 2009 Liverpool Oncology Data Base 1992-2006 |
35/41 |
|
Werschnik et al 2002 Germany 1958-1993 |
54/31 |
|
Norregaard et al 1996 Denmark 1960-1980 |
19/23 |
|
Shields et al 2011 1970-2003 Wills Eye Hospital |
196/186 |
- Incidence appears to be higher in Caucasians than in Asian, Hispanic, or Black patients. This is based on the 382 conjunctival melanomas treated at Wills only 6% of the patients were being Asian, Hispanic or Black – the remainder were Caucasian. (combined two bullet points) This is in addition to the study of 168 conjunctival melanomas from 1992-2003, identified through the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registries, the annual age adjusted incidence rates showed about 50% of the tumors in white patients (Hu, Yu 2008).
- African Americans are affected with 0.2/million more than would be expected based on incidences of skin/uveal melanoma in that group (Shildkrot, Wilson 2010).
- Mortality rate at 5-year 8% (Esmaeli, Roberts et al. 2012)<
- Risk of regional node metastasis at 10-year 11% (Esmaeli, Roberts et al. 2012)
- Risk of metastatic rate 10-year 19% (Esmaeli, Roberts et al. 2012)
History
- Risk factors not established except that 57%–76% of conjunctival melanoma arise from primary acquired melanosis (PAM, Figure 1).
- De novo or from nevi is much rarer. (Wong, Nanji et al. 2014)
- Data regarding association between UV light and conjunctival melanoma equivocal (Wong, Nanji et al. 2014)
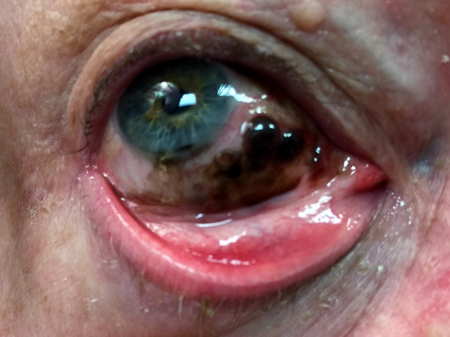
Figure 1. Conjunctival melanoma arising from PAM.
Clinical features
- Typical presentation: elevated pigmented nodule affecting bulbar, palpebral, forniceal, or caruncle conjunctiva; latter 3 increase likelihood of malignancy
- “Conjunctival” melanomas can actually develop on and be limited to the corneal epithelium (Tuomaala, Aine 2002).
- Amelanotic/minimal pigmented in 19% of cases (Figure 2).
- Typically associated with PAM.
- Other clinical features: increased vascularity, immobility, and absence of cysts (Shildkrot, Wilson 2010)
- Most frequent locations bulbar and limbal > cornea > palpebral, forniceal and caruncular. (Oellers, Karp 2012)
- Should invert the upper lid and check lower lid fornix to ensure no “hidden” lesions.
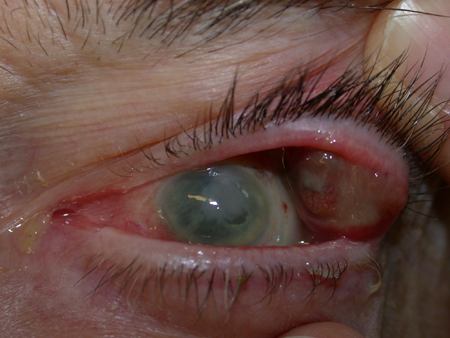
Figure 2. Recurrent amelanotic conjunctival melanoma of the palpebral conjunctiva in a blind eye.
Testing
- Full history and physical exam including palpating for regional lymph nodes.
- Adjunctive imaging (OCT, ultrasound) can be useful. (Oellers, Karp 2012)
- OCT in pigmented lesions can demonstrate hyper-reflectivity of the basal epithelium in PAM and nevi cysts thereby reducing the likelihood of conjunctival melanoma (Oellers, Karp 2012)
- Photography to document lesion changes
- Literature has described use of dermoscope for clinical evaluation of conjunctival melanoma. Of limited benefit. (Li, Xin 2014)
- Mainstay of diagnosis is by histology using excisional biopsy techniques.
- In a series of 85 conjunctival melanomas treated in Helsinki, Finland, tumor thickness of 2mm or greater predisposed to metastatic disease (Tuomaala, Kivela 2004).
- Some cases maybe indeterminate. These cases benefit from immunohistochemistry markers.
- S100 is sensitive but not specific for conjunctival melanoma. HMB-45 is less sensitive but more specific. Wilms tumor gene 1 protein expression useful to distinguish between benign and malignant. Others including Ki-67 and Bc1-2 recently shown to be consistent immunohistochemical marker for melanocytic conjunctival tumors. (Lim, Madigan et al. 2013)
- Fluorescence in situ hybridization (FISH) has been shown to be useful in equivocal cases for diagnostic purposes. (Mudhar, Smith et al. 2013)
- Amelanotic melanoma can be differentiated from squamous conjunctival neoplasia by demonstrating expression of MART-1 – melanocyte antigen marker (Betts, Espana 2015).
- Recent evidence looking at genetic alterations in conjunctival melanoma showed 47% of had presence of NRAS and BRAF mutations. Suggests conjunctival melanoma more related to cutaneous rather than uveal melanoma. May impact therapeutic options. (Griewank, Westekemper et al. 2013)
Testing for staging, fundamental impairment
- Staging based on the AJCC (American Joint Committee on Cancer) (Shields, Kaliki et al. 2012), (Yousef, Finger 2012), (Wong, Nanji et al. 2014)
- Tis melanoma confined to conjunctival epithelium (i.e., PAM with atypia)
- T1 conjunctival melanoma on bulbar conjunctiva
- T2 conjunctival melanoma on non-bulbar conjunctiva (palpebral, forniceal, caruncular)
- T3 conjunctival melanoma with local invasion including eye, eyelid, orbit, sinus)
- T4 invasion locally to the CNS
- N regional lymph nodes
- M distant metastasis
- PET scan has been reported to identify preauricular regional tumor extension (Damian, Gaudiano 2013).
-
Whole body PET/CT imaging was performed in fourteen patients with clinical stage T3 and greater disease and only one had evidence of systemic metastasis – to the liver, lung, peritoneum, and lumbar spine (Kurli, Chin 2008).
Risk factors
- Primary acquired melanosis
- De novo or from a conjunctival nevus.
- Of the 382 conjunctival melanomas treated at Wills Eye Hospital from 1970-2003, 74% (n=284) developed in primary acquired melanosis, 7% (n=26) developed from nevi and the remainder were apparently de novo tumors (Shields, Markowitz 2011).
- One patient had a history of acquired immunodeficiency syndrome – HIV infection does not seem to be a predisposing factor.
- In that series, extent of PAM was 1 to 3 clock hours (38%), 4 to 6 clock hours (32%), 7 to 9 clock hours (12%), and 10 to 12 clock hours (19%) – extent of PAM does not seem to be a predisposing factor.
- No patient in that series had undergone previous periocular radiotherapy – prior radiotherapy does not seem to be a predisposing factor.
- Iris color in that series was blue in 205 patients (54%) and brown in 137 patients (36%) – the remainder had iris color described as hazel – iris color does not seem to be a predisposing factor.
- Conjunctival melanoma from de novo carries higher risk of metastasis and death versus those arising from PAM or nevus. (Shields, Markowitz et al. 2011)
- Shields has shown that conjunctival melanomas with AJCC stages T2 or T3 have higher rates of local recurrence, regional lymph node metastasis, distant metastasis and death versus those with T1 staging. (Shields, Kaliki et al. 2012).
- Esmaeli et al have not shown this. They notes that tumor location bulbar (T1) versus non-bulbar (T2 or T3) was not significantly correlated with regional lymph node metastasis or death. Rather, histologic features such as thicker tumor, ulceration, and higher mitotic rate correlated with regional lymph node metastasis. (Esmaeli, Roberts et al. 2012) (delete bullet point) They also reported that Hhistologic features such as lymphovascular spread, epitheloid cell type and microsatellitosis are correlated with death. (Esmaeli, Roberts et al. 2012)
- Amelanotic melanoma can arise in amelanotic PAM (Jay, Font 1998).
- One study looked at transketolase-like-1-gene (TKTL1) protein levels and these were elevated in malignant ocular adnexal tumors including conjunctival melanoma. Enhanced expression of TKTL1 in specimens correlated with increased tumor recurrence rate. (Lange, Tisch-Rottensteiner et al. 2012)
Differential diagnosis
- Primary Aquired Melanosis (PAM)
- 4 features
- Inhomogeneous pigmentation, unilateral, acquired dusting, waxing, and waning
- Females > males
- Whites (96% of cases) > Non-Whites
- PAM with atypia can become conjunctival melanoma.
- Need histological evaluation to differentiate
- Nevus
- 4 features
- Long-term presence
- Variable pigmentation
- Often cysts
- Change with hormonal surges (pregnancy or puberty)
- Can be amelanotic and usually unilateral
- Complexion-associated melanosis
- 4 features
- Bilateral
- Dusted pigment
- Around limbus
- Seen in darkly pigmented individuals
- Can form conjunctival microfolds
- Can increase in size with age
- Other nonmelanocytic pigmented lesions
- Pigmented squamous cell carcinoma
- Adrenochrome deposition
- Foreign body
- Silver deposits from eye drops
- Conjunctivochalasis
- Surgical scar
- Extraocular extension of a uveal melanoma can appear clinically as a pigmented lesion of the conjunctiva and sclera (Phelps, Surapaneni 2015).
- Cutaneous melanoma can metastasize to the conjunctiva and present as a conjunctival mass (Shields, Shields 2004) (Oellers, Karp 2012).
Patient management: treatment and follow-up
- Recurrence from multifocal disease and foci characterize the natural clinical course of this disease, despite apparently effective primary surgical excision of the mass.
- There are three clinical approaches to addressing this problem:
- Adjuvant chemotherapy can be administered after surgical excision, with topical mitomycin-C. This can effectively treat subclinical foci of disease, with risks to the ocular surface.
- Map biopsies may be help identify in situ extension beyond clinically evident disease. They are particularly important for amelanotic lesions where primary excision may not be adequate. Map biopsies represent random sampling and recurrences appear without a pattern of contiguous growth. Map biopsies may identify subclinical disease, and indicate need for adjuvant therapy, otherwise may yield false negative.
- Vigilant clinical monitoring with vigorous cryotherapy to focus on recurrence.
- Primary excisional biopsy and adjuvant therapy is preferred – among 139 conjunctival melanomas treated at the University of Copenhagen, incisional biopsy alone led to higher rates of metastasis (Gombos 2014).
- Risk factors for Local Recurrence
- 40% over mean of 2.4 years
- Thickness of tumor
- Subsequent local recurrences
- Incomplete excision at time of surgery
- Nonlimbal location
- Nonlimbal recurrence: Plica semilunaris or nonbulbar (fornix, conjunctiva, caruncle, or eyelid) greater than epibulbar
- In a review of 27 patients treated at MD Anderson Cancer Center from 1962-1999, 11 patients (41%) developed regional lymph node metastases, 1.5-6.0 years (mean 3.2 years) after the initial diagnosis. In seven patients distant metastasis developed without evidence of nodal spread (Esmaeli, Wang 2001) .
- Metastatic frequency 734 cases over 5 large studies 19% over a mean interval of 3.4 years
- When stratified according to AJCC classification 5 year 11%–42% and 10 year 20%–52%
- Melanoma associated mortality frequency 734 cases over 5 large studies 18% after surgical resection with tumor-free margins over mean interval of 4.9 years.
- When stratified according to AJCC classification 5- and 10-year melanoma associated mortality 5%–23% and 14%–20% respectively
- Risk factors for metastatic disease (Oellers, Karp 2012):
- Tumor origin de novo
- Palpebral location
- Nodular tumor
- Orbital invasion at presentation
- Pathologic tumor margins
- Tumor thickness Breslow (> 1–2 mm)
- Difficulty in clinically recognizing amelanotic PAM and secondary amelanotic melanoma can result in significantly more advanced disease at presentation (Paridaens, McCartney 1992).
- Risk factors for death: (Oellers, Karp 2012)
- Tumor origin de novo
- Fornix, caruncle, plica location
- Nodular tumor
- Tumor thickness Breslow (> 1–2 mm)
- History of local recurrence is also a risk factor for metastatic disease (Shildkrot, Wilson 2010).
- Of note caruncle location has 50% mortality at 3 years (Shildkrot, Wilson 2010).
- One case in literature of spontaneous regression of biopsy proven melanoma (Miller, Cook et al. 2014)
Medical therapy
- Medical treatment is an adjuvant therapy following surgical excision to improve local control.
- Can be systemic of local treatment.
- Sensitivity to chemotherapeutic agents has been studied in two conjunctival melanoma cell lines (CRMM-1 and CRMM-2).
- Cisplatinum and mitomycin-C effectively inhibit tumor growth in these cell lines and imatinib and mitomycin is effective combination therapy (Westekemper H, Freistuehler 2012).
- Topical Agents
- Suggest punctual plugs to increase local absorption and diminish chances of punctal stenosis.
- Most studied topical agent is Mitomycin-C (MMC) – dosage is similar to treatment of PAM – 0.02% or 0.04% qid for 1–3 week cycles
- Side-effects of topical MMC: epithelial defects, limbal stem cell deficiency, tearing, hyperemia, pain, punctuate epithelial defects, disciform keratitis, contact dermatitis.
- Interferon alpha-2b (IFN-alpha-2b) alternative that can be used in a topical, subconjunctival and perilesional fashion; fewer side effects compared to MMC. Dose for topical application: 1 million IU qid for 1 month after clinical resolution of lesion
- Efficacy: MMC saw 60.7% remission rate for primary use and 68.8% when used as adjuvant. Interferon alpha-2b achieved 87.5% remission across primary and adjuvant applications (Tzortzi, 2025)
- Immunotherapy
- Can be used in locally extensive disease, metastatic disease or for recurrences
- Various regimens: PD-1 inhibitor monotherapy with nivolumab or pembrolizumab, ipilimumab (CTLA-4 inhibitor) and nivolumab for several cycles followed by nivolumab monotherapy
- In literature, rate of objective response to ICI therapy was 63%, and for the subset of patients with local disease only, the objective response rate was 70% (Esmaeli 2025)
Radiation
- In general conjunctival melanoma is not radiosensitive. Radiotion has been used in the past but should not be used as sole therapy.
- From 1943-1973, 26 conjunctival melanomas were treated at Moorfields Hospital in London with radiotherapy as primary treatment and surgery reserved for failures. Complications were noted in 7 of the 26 patients, including cataract, glaucoma and keratopathy (Lederman, Wybar 1984).
- Radiation should be used for:
- Deep sclera invasion following primary excision
- Recurrent disease unresponsive to other therapies
- Palliation for extensive unresectable disease
- Patients who cannot tolerate surgery
- External beam radiotherapy, proton beam radiation, brachytherapy are all options
Surgery
- Surgery is the mainstay of treatment
- Wide local excision and biopsy with “no-touch” technique and cryotherapy to reduce concerns of seeding, tumor dissemination and recurrence
- Wide excisional biopsy is preferred to incisional but in cases of extensive lesions incisional biopsy may be performed initially.
- Published data on no touch show only positive trend that is not significant for a better outcome but no disadvantage. (Oellers, Karp 2012)
- Cryotherapy in combination with surgical excision at initial treatment significantly reduces recurrence rates as compared to surgical excision alone but is of no benefit for prevention of metastatic disease. (Lim, Madigan et al. 2013)
- Technique of wide local excision includes:
- 4-mm wide surgical margins marked
- Surgical excision with no touch
- Specimen oriented in consultation with pathologist for margin control
- Absolute alcohol in involved areas of cornea for 1 minute (corneal epitheliectomy)
- Corneal cells debrided (maintain intact Bowman’s layer) and sent for evaluation
- Cryotherapy (double freeze) to surgical margins (conjunctiva is elevated from underlying sclera to reduce damage)
- Amniotic membrane graft sutured in place +/- symblepharon ring
- Other options include direct closure for small defects or use of allografts (e.g., buccal mucosal).
- Enucleation and exenteration have shown no improvement in survival in conjunctival melanoma and should be performed as palliation for orbital invasion or complete conjunctival involvement (Wong, Nanji et al. 2014)
- Sentinel lymph node biopsy (Pfeiffer, Savar et al. 2013), (Nijhawan, Ross et al. 2004), (Wong, Nanji et al. 2014), (Mendoza, Grossniklaus 2015)
- Debate among clinicians about the use of sentinel lymph node biopsy
- Relatively safe procedure
- Main risk is mild temporary weakness of facial nerve/branches
- Main issue is risk of false negative results (i.e., negative lymph node biopsy but with subsequent nodal disease involvement).
- Current indications for sentinel lymph node biopsy for conjunctival melanoma are tumors greater than or equal to 2 mm in histologic thickness and/or histologic ulceration.
- Some advocate done at time of surgical resection of tumor while others suggest secondarily after the initial surgical resection.
Other management considerations
- Use of permanent margins is key as frozen section control of margin is unreliable.
- Surgical specimen handling
- Flatten the conjunctival specimen; avoid curling of the borders of the specimen and thus tangential cutting
- Direct communication with the pathologist is key to avoid pitfalls in specimen handling.
- If margins are positive on the excisional biopsy specimen, go back and remove more.
- May have to remove parts of the eyelid, caruncle, canaliculi to adequately address the full extent of melanoma involvement.
- All patients should be sent to an oncologist for systemic workup
- Genetic Therapies
- Conjunctival melanoma has some similar genetic mutations to cutaneous melanoma (and distinctly different to uveal melanoma).
- Genetic targets in conjunctival melanoma include BRAF, KIT and NRAS.
- Therapies targeted at inhibiting these genes (e.g., BRAF) have been tried in conjunctival melanoma with promise.
Common treatment responses, follow-up strategies
- Follow up after treatment every 4–6 months for the lifetime of patient
- Metastatic routes include lymphatic, hematogenous as well as direct extension into eyelids, orbit, sinuses, nasolacrimal system and CNS
Preventing and managing treatment complications
- Complications of surgery: symblepharon, nonhealing epithelial defects, hyphema, limbal stem cell deficiency, corneal scarring, infection, diplopia (Wong, Nanji et al. 2014). Amniotic membrane grafts are useful in preventing many of these complications.
- Complications of cryotherapy: damage to conjunctiva, cornea, iris, conjunctival chemosis, subconjunctival hemorrhage, sclera/corneal tissue loss, ciliary body damage and hypotony, damage to eyelids, uvea, extraocular muscles, dry eyes, sclera melt (Wong, Nanji et al. 2014). Ideally best way to prevent these complications is to ensure that one is not aggressive with cryo, that margins are preferentially treated and that the conjunctiva is lifted away from the sclera/cornea during treatment.
- Complications of topical chemotherapy such as mitomycin-C include keratopathy, limbal stem cell disease, and persistent corneal erosions. Should use caution and close observation during treatment (Ditta, Shildkrot et al. 2011)
- Complications of radiotherapy: dry eyes, loss of lashes, focal cataracts, limbal stem cell deficiency, keratinization of conjunctiva, corneal complications. (Wong, Nanji et al. 2014)
Disease-related complications
- Vision loss and chronic ocular irritation can occur from multiple causes after surgery and adjuvant treatment.
- Local invasion into ocular adnexal structures including eyelids, cornea, sclera extension, nasolacrimal duct, orbital extension, sinus, CNS extension
- Intraocular extension can be facilitated by corneal and scleral wounds or filtration surgery (Samara, Eagle 2015).
- Tumor excision with sclerokeratectomy can predispose to intraocular invasion with tumor recurrence (Wenkel, Rummelt 2000).
- Six cases of epistaxis and epiphora have been reported with conjunctival melanoma extending into the nasolacrimal duct (Missotten, Gambrelle 2010).
- Beyond extension into the nasopharynx, a case of gastric metastases from conjunctival melanoma has been described (Cohen, Ahmadi-lari 2007).
- Metastatic disease to regional lymph nodes, chest, liver, spleen, etc.
- Of 40 conjunctival melanomas treated from 1993-2006 four patients died of metastases, all with caruncular disease (Damato, Coupland 2009).
References and additional resources
- Aronow, M.E. and Singh, A.D., 2013. Radiation therapy: conjunctival and eyelid tumors. Developments in ophthalmology, 52, pp. 85-93.
- Betts RR, Espana EM, Margo CE: Amelanotic melanoma arising within conjunctival melanocytic intraepithelial neoplasia sine pigmento. Ophthalmology 2015; 122:2178.
- Cohen VM, Ahmadi-lari S, Hungerford JL. Gastric metastases from conjunctival melanoma. Melanoma Res 2007; 17(4):255-6.
- Damato B, Coupland S. An audit of conjunctival melanoma treatment inLiverpool. Eye Lond 2009;23:801-9.
- Damian A, Gaudiano J, Engler H, et al: (18)F-FDG PET-CT for staging of conjunctival melanoma. World J Nucl Med 2013;12(1):45-7.
- Ditta, L.C., Shildkrot, Y. and Wilson, M.W., 2011. Outcomes in 15 patients with conjunctival melanoma treated with adjuvant topical mitomycin C: complications and recurrences. Ophthalmology, 118 (9), pp. 1754-1759.
- Dravitman-Storobinsky O, Cohen Y, Frenkel S, et al: Lack of oncogenetic GNAQ mutations in melanocytic lesions of the conjunctiva as compared to uveal melanoma. Invest Ophthalmo Vis Sci 2010; 51:6180.
- Esmaeli B, Ogden T, Nichols M, et al. Rate of response to immune checkpoint inhibitor therapy in patients with conjunctival melanoma. Melanoma Res. 2025;35(2):130-144.
- Esmaeli B, Roberts, D., Ross, M., Fellman, M., Cruz, H., Kim, S.K. and Prieto, V.G., 2012. Histologic features of conjunctival melanoma predictive of metastasis and death (an American Ophthalmological thesis). Transactions of the American Ophthalmological Society, 110, pp. 64-73.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology 2001;108(11): 2101-5.
- Gear H, Williams H, Kemp E, Roberts F. BRAF Mutations in conjunctival melanoma. Invest Ophthalmol Vis Sci 2004; 45:2484.
- Griewank, K.G., Westekemper, H., Murali, R et al.. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clinical cancer research : an official journal of the American Association for Cancer Research, 19 (12), pp. 3143-3152.
- Goldenberg-Cohen N, Cohen Y, Rosenbaum E, et al. T1799A BRAF Mutations in Conjunctival Melanocytic Lesions. Invest Ophthalmol Vis Sci 2005; 46:3027.
- Gombos DS: Conjunctival melanoma – clinical pearls now, hope for the future. JAMA Ophthalmol 2014; 132:1432.
- Harooni, H., Schoenfield, L.R. and Singh, A.D., 2011. Current appraisal of conjunctival melanocytic tumors: classification and treatment. Future oncology (London, England), 7 (3), pp. 435-446.
- Hu D, Yu G, McCormick SA, Finger PT: Population-based incidence of conjunctival melanoma in various races and ethnic groups and comparison with other melanomas. Am J Ophthalmol 2008; 145:418-23.
- Jain P, Finger PT, Filì M, et al. Metastatic conjunctival melanoma: a multicentre international study. Br J Ophthalmol. 2025;109(6):652-659.
- Jakobiec FA, Brownstein S, Wilkinson RD, et al. Adjuvant cryotherapy for focal nodular melanoma of the conjunctiva. Arch Ophthalmol 1982;100(1):115-18.
- Jay V, Font R: Conjunctival amelanotic melanoma arising in primary acquired melanosis sine pigmento. Ophthalmology 1998; 105:191.
- Kurli M, Chin K, Finger P: Whole-body 18 FDG PET/CT imaging for lymph node and metastatic staging of conjunctival melanoma. Br J Ophthalmol 2008; 92:479-82.
- Lange, C.A., Tisch-rottensteiner, J., Bohringer, D., Martin, G., Schwartzkopff, J. and Auw-haedrich, C., 2012. Enhanced TKTL1 expression in malignant tumors of the ocular adnexa predicts clinical outcome. Ophthalmology, 119 (9), pp. 1924-1929.
- Larsen A, Dahmcke CM, Dahl C, et al: A retrospective review of conjunctival melanoma presentation, treatment, and outcome and an investigation of features associated with BRAF mutations. JAMA Ophthalmol 2015; 133:1295-1303.
- Lederman M, Wybar K, Busby E. Malignant epibulbar melanoma: natural history and treatment by radiotherapy. Br J Ophthalmol 1984; 68: 605–617.
- Li, K. and Xin, L., 2014. Palpebral conjunctiva melanoma with dermoscopic and clinicopathological characteristics. Journal of the American Academy of Dermatology, 71 (2), pp. e35-7.
- Lim, L.A., Madigan, M.C. and Conway, R.M., 2013. Conjunctival melanoma: a review of conceptual and treatment advances. Clinical ophthalmology (Auckland, N.Z.), 6, pp. 521-531.
- Mendoza, P.R. and Grossniklaus, H.E., 2015. Sentinel lymph node biopsy for eyelid and conjunctival tumors: what is the evidence? International ophthalmology clinics, 55 (1), pp. 123-136.
- Mihajilovic M, Vlajkovic S, Jovanvic P, Stefanovic V: Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol 2012; 5:739.
- Miller, C.V., Cook, I.S., Jayaramachandran, R. and Tyers, A.G., 2014. Spontaneous regression of a conjunctival malignant melanoma. Orbit (Amsterdam, Netherlands), 33 (2), pp. 139-141.
- Missotten GS, Gambrelle J, de Wolff-Rouendaal D, de Keizer RJW. Epistaxis or epiphora as a sign for extension of a conjunctival melanoma. A series of six patients with nasolacrimal recurrence. Br J Ophthalmol 2010; 94(10):1328-31.
- Mudhar, H.S., Smith, K., Talley, P., Whitworth, A., Atkey, N. and Rennie, I.G., 2013. Fluorescence in situ hybridisation (FISH) in histologically challenging conjunctival melanocytic lesions. The British journal of ophthalmology, 97 (1), pp. 40-46.
- Nijhawan, N., Ross, M.I., Diba, R., Ahmadi, M.A. and Esmaeli, B., 2004. Experience with sentinel lymph node biopsy for eyelid and conjunctival malignancies at a cancer center. Ophthalmic plastic and reconstructive surgery, 20 (4), pp. 291-295.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol 1996; 234(9):569-72.
- Oellers, P. and Karp, C.L., 2012. Management of pigmented conjunctival lesions. The ocular surface, 10 (4), pp. 251-263.
- Paridaens AD, McCartney AC, Hungerford JL: Multifocal amelanotic conjunctival melanoma and acquired melanosis sine pigmento. Br J Ophthalmol 1992; 76:163-5.
- Phelps PO, Surapaneni KR, Nehls SM, Altaweel MM: Uveal melanoma presenting as a pigmented conjunctival lesion. Ophthalmology 2015; 122:1652.
- Populo H, Soares P, Rocha AS, et al. Evaluation of the mTOR pathway in ocular (uvea and conjunctiva) melanoma. Melanoma Res 2010; 20:107.
- Pfeiffer, M.L., Savar, A. and Esmaeli, B., 2013. Sentinel lymph node biopsy for eyelid and conjunctival tumors: what have we learned in the past decade? Ophthalmic plastic and reconstructive surgery, 29 (1), pp. 57-62.
- Samara WA, Eagle RC, Lally SE, Shields CL: Conjunctival melanoma with intraocular extension. Ophthalmology 2015; 122:2335.
- Shields, C.L., Kaliki, S., Al-Dahmash, S.A., Lally, S.E. and Shields, J.A., 2012. American Joint Committee on Cancer (AJCC) clinical classification predicts conjunctival melanoma outcomes. Ophthalmic plastic and reconstructive surgery, 28 (5), pp. 313-323.
- Shields, C.L., Markowitz, J.S., Belinsky, I., Schwartzstein, H., George, N.S., Lally, S.E., Mashayekhi, A. and Shields, J.A., 2011. Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases. Ophthalmology, 118 (2), pp. 389-95.e1-2.
- Shields JA, Shields CL, Eagle RC, Raber IM: Conjunctivl metastasis as initial sign of disseminated cutaneous melanoma. Ophthalmology 2004; 111:1933-4.
- Shields, C.L., Shields, J.A., Gunduz, K., Cater, J., Mercado, G.V., Gross, N. and Lally, B., 2000. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Archives of ophthalmology (Chicago, Ill.: 1960), 118 (11), pp. 1497-1507.
- Shields CL, Silva AMV, Laiton A, et al. Conjunctival melanoma: Insights into classification, outcomes, and biomarkers. Clin Dermatol. 2024;42(1):46-55.
- Shildkrot, Y. and Wilson, M.W., 2010. Conjunctival melanoma: pitfalls and dilemmas in management. Current opinion in ophthalmology, 21 (5), pp. 380-386.
- Spendlove HE, Damato BE, Humphreys J, et al. BRAF mutations are detectable in conjunctival but not uveal melanomas. Melanoma Res 2004;14(6):449.
- Tuomaala S, Aine E, Saari KM, Kivela T: Corneally displaced malignant conjunctival melanoma. Ophthalmology 2002; 109:914.
- Tuomaala S, Kivela T: Metastatic pattern and survival in disseminated conjunctival melanoma: Implications for sentinel node biopsy. Ophthalmology 2004; 111:816.
- Tzortzi P, Tzamalis A, Mataftsi A, Tsinopoulos I. Topical chemotherapy for the treatment of melanocytic conjunctival tumors: An updated review. Eur J Ophthalmol. Published online June 19, 2025:11206721251351152.
- Wenkel H, Rummelt V, Naumann GOH: Malignant melanoma of the conjunctiva with intraocular extension. Arch Ophthalmol 2000; 118:557-560.
- Werschnik C, Lommatzsch PK. Long-term follow-up of patients with conjunctival melanoma. Am J Clin Oncol 2002;25(3): 248-55.
- Westekemper H, Freistuehler M, Anastassiou G, et al: Chemosensitivity of conjunctival melanoma cell lines to single chemotherapeutic agents and combinations. Br J Ophthalmol 2012; 96:591-6.
- Wong, J.R., Nanji, A.A., Galor, A. and Karp, C.L., 2014. Management of conjunctival malignant melanoma: a review and update. Expert review of ophthalmology, 9 (3), pp. 185-204.
- Yousef, Y.A. and Finger, P.T., 2012. Predictive value of the seventh edition American Joint Committee on Cancer staging system for conjunctival melanoma. Archives of ophthalmology (Chicago, Ill.: 1960), 130 (5), pp. 599-606.
Financial disclosures
Financial Disclosures
Reviewers
Victoria North – No disclosures
