Endoscopic, Endonasal, or Transnasal Dacryocystorhinostomy (EndoDCR)
Updated July 2024
Robert G. Fante, MD, FACS; Richard C. Allen, MD, Phd
Over the past 25 years, there has been renewed interest in the transnasal approach to dacryocystorhinostomy (DCR) with many variations in surgical technique that are united in their avoidance of an external incision. The use of an endoscope with video magnification can facilitate recognition of surgical landmarks, but many surgeons find that many of the surgical steps are more easily performed with headlight observation. This article reviews the transnasal approach, with or without endoscopic assistance, to acquired epiphora related to the nasolacrimal duct.
Goals
Primary:
- Sustained relief from symptomatic epiphora
- Cure for acute or chronic dacryocystitis
- Postsurgical patient satisfaction and relative value has been established by quality of life studies (Jutley, Eye 2013).
Indications and contraindications
Indications are similar to those for external DCR:
- Tearing caused at least in part by partial or complete nasolacrimal duct (NLD) obstruction
- Acute dacryocystitis during or after antibiotic treatment
- NLD obstruction with chronic dacryocystitis
- Tearing without identifiable cause after complete workup (see comments below in Clinical Examination section)
EndoDCR might have advantages over external DCR in some circumstances:
- Useful in younger patients and patients with flat nasal bridge requiring dacryocystorhinostomy (DCR) when any visible scar is undesirable, however, in some patients with a wide central midface, intervening ethmoid air cells between the lacrimal sac and nasal cavity might make endoDCR more challenging.
- To avoid disturbing a stable, asymptomatic medial canthal deformity from prior trauma, or surgery such as skin tumor resection/radiation therapy
Relative contraindications:
- Complete canalicular obstruction, however, endoDCR can be combined with CDCR/Jones tube; see below.
- Management of lacrimal sac tumor, lacrimal system stone, or foreign body is possible, but might be more difficult via endonasal route than external DCR, at least for novice surgeons.
- Can be more difficult in setting of trauma/previous trauma repair when fractures or bone hypertrophy distort anticipated anatomy; imaging is recommended.
- Can be more difficult in setting of nasal septal deviation, unless concurrent septoplasty performed
Preprocedure evaluation
- Complete ophthalmic/medical history with attention to history of
- Topical glaucoma/anti-viral therapy
- Ocular surface disease
- Herpes simplex virus (HSV) infection
- Paranasal sinus disease/surgery
- Facial trauma/surgery/radiation therapy
- Acetylsalicylic acid (ASA)/anticoagulant therapy
- Lacrimal sac tumor
- Granulomatosis with polyangiitis
- Sarcoidosis
- Facial neuropathy
- Allergic fungal sinusitis
- Radioactive iodine therapy
- Clinical examination (see related article, Evaluation of Epiphora)
- Oculofacial external exam
- Slit lamp biomicroscopy of eyelids, puncta, tear film, and anterior segment
- Complete lacrimal evaluation
- Canalicular probing/irrigation
- Jones’ tests
- Nasal examination (office endoscopy preferred)
- Especially note location of nasal septum, size and configuration of middle turbinate and uncinate process (Figures 1 and 2), presence of polyps or other pathology in the inferior and middle meatus areas.
- DCR has been studied in patients with epiphora who have a patent lacrimal outflow system to clinical testing and no other cause for epiphora:
- 94% success among 51 patients (O’Donnell, Clin Experiment Ophthalmol 2001)
- Success in these patients has been correlated with the single drop Jones test, especially in the presence of delayed fluorescein dye disappearance and canalicular reflux on irrigation (O’Donnell OPRS 2007)
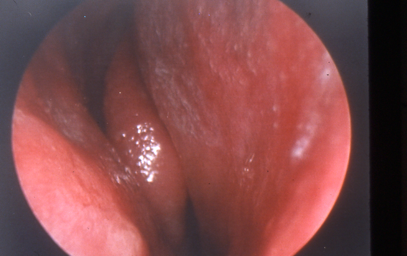
Figure 1. Narrow middle meatus and large turbinate.
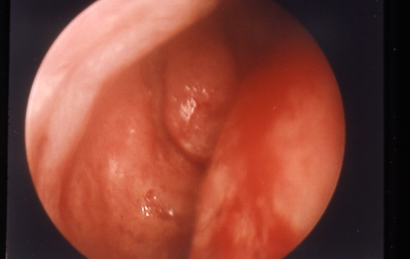
Figure 2. Wide middle meatus and small turbinate with prominent uncinate process.
- Potential imaging options [uncommonly performed in routine cases among ASOPRS members (Nagi, OPRS 2010)]
- Orbit/sinus computed tomography (CT)/magnetic resonance imaging (MRI) scan
- Dacryocystography
- Radionuclide dacryoscintigraphy
Alternatives to procedure
- Observation
- Medical therapy of dacryocystitis, if present
- Lacrimal probing with Mitomycin C
- Standard probing followed by irrigation of 0.5–1 cc mitomycin C 0.2 mg/ml into the nasolacrimal duct, held there with nasal packing for 10 minutes, followed by irrigation of the conjunctival fornices with BSS
- 32/36 eyes (89%) had postoperative patent NLD at 9 months after probing with mitomycin C, but only 9 (25%) were symptom-free (Tsai, Ophthalmology, 2002).
- Balloon dacryoplasty
- Anterograde: 69% improved among 102 cases with at least 12 months follow-up (McCullough, Clin Radiol 2001).
- Retrograde: In a study of 52 cases, 69% failure among complete NLDO and 17% failure for partial NLDO (Fenton, Eye 2001)
- Balloon dacryoplasty plus silicone intubation
- 73% improvement among 30 patients at 1 year (Kuchar, BJO 2001)
- Polyurethane stent (available outside US)
- 55% long-term improvement among 426 patients and recommended by authors for unsuitable surgical candidates (Lanciego, J Vasc Interv Radiol 2003)
- Dacryocystectomy
- External DCR
- Conjunctivodacryocystorhinostomy (CDCR)
- Best alternative when there is severe canalicular obstruction
- Can be performed in conjunction with endoDCR (Trotter, Ophthalmology 2000)
- Botulinum toxin injection into the lacrimal gland
- Munk score improved in a study of 17 patients who underwent botulinum toxin injection into the lacrimal gland rather than surgery for lacrimal outflow obstruction (Ziahosseini, Eye 2015)
Techniques used with procedure
Simulator training (Weiss, OPRS 2009) or apprenticeship with an experienced surgeon might be appropriate for surgeons beginning to use these techniques.
Anesthesia:
- EndoDCR is most easily performed under general anesthesia. An oral endotracheal tube (ET tube) interferes least with the surgeon’s hands and instruments, but a standard ET tube or a laryngeal mask airway (LMA) is also usually satisfactory. Pooling of blood in the posterior pharynx is likely and should be discussed with anesthesiologist.
- Several papers describe successful local anesthesia alone for endoDCR (see below). However, the lack of airway protection raises concern in the event of significant intraop bleeding.
- Local infiltration using lidocaine with epinephrine of the region of the uncinate process of the lateral wall of the nasal cavity from the anterior root of the middle turbinate inferiorly for about 2 cm under direct headlight observation or endoscopy (1.5-inch 25-gauge needle or 22-gauge spinal needle work well). This is followed by placement of nasal packing soaked in 0.5% oxymetazoline (two 0.5″ x 3″ cottonoids usually suffice per side) for 5–7 minutes.
No prep is necessary, but standard draping is helpful. Commonly used instruments include 30-degree endoscope/camera/video tower, Frasier suction, nasal speculum (medium), Freer or Cottle elevator, Kerrison rongeurs (small and medium), small Takahashi or Blakesley forceps, 4-mm osteotome and mallet, 66 Beaver blade or sickle knife or 12 blade on long handle, punctal dilator, Bowman probes, silicone intubation of surgeon’s choice.
For right-handed surgeons, both sides are most easily accessible by standing at the patient’s right shoulder. This position is easier because the nondominant hand holds the endoscope or nasal speculum, leaving the dominant hand free for surgical instruments. The Mayo stand can be positioned at the head of the bed or over the patient, but in this location is at risk for contamination by the surgeon leaning over to look up the nose with the headlight. The video screen is positioned over the patient’s left shoulder. Suction and endoscope cables can be at the patient’s chest or on the Mayo stand at the head of the bed. Reverse for left-handed surgeons.
Depending on variability in anatomy and intraoperative bleeding, the surgery can be easier either with the view using an endoscope or the view directly with a headlight. Surgeons can readily have both options available to them for their cases.
Variations in the space around the middle turbinate and uncinate process may affect surgical technique. See Figures 1 and 2 for examples of narrow and wide middle meatus.
Surgical technique:
- With headlight observation and nasal speculum or the endoscope, incise nasal mucosa with Cottle or Freer about 0.5 cm anterior to the uncinate process and the root of the middle turbinate, starting superiorly and extending inferiorly along the path of the lacrimal sac for about 1.5 cm. Try to avoid injury to the nasal septal mucosa from the speculum or other instruments. The endoscope can be used for primary observation or at any time to double check anatomy.
- Still using headlight observation and nasal speculum or the endoscope, Kerrison rongeurs are positioned to bite the bone and mucosa just anterior to the middle turbinate, thereby exposing the lacrimal sac. Typically the deep end will pass lateral to or under the middle turbinate (which might require infracture) and the proximal cutting end will be positioned along the nasal mucosal incision. Multiple bites are taken, removing bone and mucosa and almost always exposing the lacrimal sac, which is then further exposed for all or almost all of its length. Bleeding during this rhinostomy can occasionally be challenging; repacking for a few minutes with cottonoids is usually adequate. For assistance with the anatomy, a retinal light pipe positioned in the lacrimal sac might be helpful for the first few cases or as desired for difficult cases. See article by Shams for anatomy of the lateral nasal wall (Shams, Curr Opin Ophthalmol 2015).
- Although infracture of the middle turbinate is occasionally necessary, it is generally wise to avoid injury to the turbinates when possible (Gustafson, OPRS 1999) because adhesions, bleeding, and damage to the ostiomeatal complex with subsequent maxillary sinusitis can occur.
- Gentle palpation in the region of the medial canthus at the lacrimal sac will now be seen from inside the nose as bulging in the region of the osteotomy. Sometimes this is easily seen by headlight observation, but endoscopic observation is often needed. Continued bone removal to better expose the sac is then performed.
- Depending on the remaining bone, useful instruments might include the Takahashi forceps, the Cottle elevator, and the Kerrisons. Switching back and forth between direct headlight observation and endoscopic observation might be helpful. Endpoint is exposure of at least 1 cm vertically of the sac, with the superior extent of the osteotomy above the valve of Rosenmuller (checked by passing a Bowman probe horizontally into the sac from the inferior punctum). The superior-anterior edge of the osteotomy can be the most difficult due to the thickness of the bone, the awkward handling of available tools, and the concern over injury to the cribriform plate and CSF leak. The Malhotra bone nibbler (Altomed) can be helpful (Patel, OPRS 2011). In rare cases, it might be necessary to remove this bone with cautious use of the 4-mm osteotome; care must be taken to avoid superior extension of bone fracture to the cribriform plate. Alternatively, the Sonopet may be a viable option to spare the soft tissue.
- Incise the medial wall of the lacrimal sac with the 66 Beaver or sickle knife or 12 blade for the entire vertical length of the osteotomy, positioning the incision closer to the posterior edge of the osteotomy. This location permits easier removal of a central portion of the medial sac wall for biopsy or to reduce potential for the sac to reform. Remember that the blade must penetrate the periosteum around the sac as well as the mucosa and some force is needed. A Bowman probe can be used to confirm completion of the mucosal incision. Release of purulence is also reassuring.
- Examine the inside of the sac with the endoscope (30-degree might be more useful here than 0-degree) to look for typical rugae, mucosal integrity, and the occasional misplaced intracanalicular plug. If present, dacryoliths can be removed by pushing them medially with a Bowman probe or by Frazier suction or by using the Cottle or Takahashi.
- The anterior and posterior sac flaps can be subtotally removed using the small Kerrison rongeurs without any twisting motion and biopsied as needed. Removal of the flaps does not negatively impact success (Hodgson, OPRS 2014) and also serves to make reformation of the sac or sump syndrome potentially less likely.
- If desired, mitomycin C can be applied with standard techniques (see below).
- If desired, silicone intubation is performed using the surgeon’s preference of stents. However, avoid using the double diameter Stent-tube because canalicular trauma is common. A Sisler lacrimal trephine (Visitec) is useful for stenosis at the valve of Rosenmuller if needed. Avoiding any tension at the puncta, the ends of the silicone stent can be tied or first passed through a silicone button and then tied.
- Hemostasis is most often adequate from the epinephrine and oxymetazoline, so that cautery and procoagulants are not needed. Even brisk bleeding often responds to reapplication of the oxymetazoline cottonoids with pressure for a few minutes.
- If there is significant iatrogenic trauma to the middle turbinate or nasal septum, consider placement of an absorbable fibrillar nasal tampon to prevent intranasal adhesions. Alternatively, use other nasal packing of the surgeon’s choice that will be removed (see below in complications).
Alternative techniques:
- Laser-assisted endoDCR: Reports range from 65% success (Tripathi, Eye 2002) to 96% success (Velegrakis, Am J Otolaryngol 2002).
- Moore compared endoscopic DCR to Holmium:YAG laser-assisted endoscopic DCR and found success rates of 71% with the laser and 86% without it (Moore, Ophthalmology 2002) whereas Maini showed no difference between the results from the two techniques (Maini, J Laryngol Otol 2007).
- Lasers studied include holmium, potassium titanyl phosphate, carbon dioxide, and others.
- Advantages could include improved hemostasis, but lasers might be less successful due to the often smaller ostium created as well as due to thermal-induced tissue damage and fibrosis leading to subacute failure.
- Mitomycin C application to the ostium at surgery: Routine mitomycin C use presents challenges related to its safe disposal and mutagenicity.
- Mitomycin C is a natural product isolated from Streptomyces species that reduces fibrosis and mucosal proliferation after topical application. Early reports used it for 30 minutes at 0.2 mg/ml (Kao, Ophthalmology 1997) and 0.5 mg/ml for 2.5 minutes (Ugurbas, Ophthalmic Surg Lasers 1997) although many other protocols have since been reported (Marcet, Curr Opin Ophthalmol 2014).
- Laser-assisted endoDCR plus mitomycin C: 99% success among 123 eyes after at least 30 months (Camara, OPRS 2000)
- Nonlaser endoDCR plus mitomycin C: 96% success among 110 eyes after at least 6 months (Kamal, OPRS 2014); similarly, 95% success in 224 eyes at 18 months (Dolmetsch, Ophthalmology 2010).
- Although these results are impressive, a study suggests that mitomycin C might be more useful in external DCR than endoDCR (Xue, Orbit 2014).
- Balloon-assisted endoDCR: Balloons and inflators add expense and the indications are unclear.
- 9-mm endonasal balloon: 92% success in 97 cases (Figure 3) (Silbert, Orbit 2010)
- 5-mm endocanalicular balloon: 91% success in 35 cases under local anesthesia (Ragab, Otolaryngol Head Neck Surg 2011); risk of iatrogenic canalicular damage and subsequent obstruction has limited adoption of this technology (Figure 4).
- Powered endoDCR
- Ultrasonic endoscopic DCR with lacrimal stent: 93.1% anatomical and 88.6% functional success rate
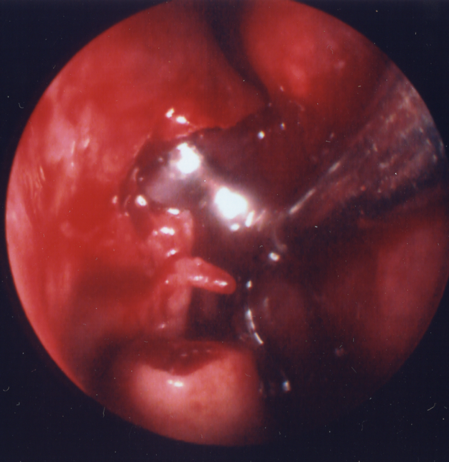
Figure 3. 9-mm endonasal balloon positioned in DCR ostium.
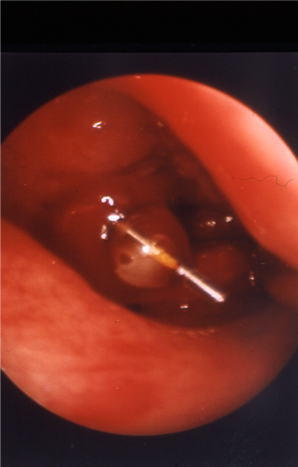
Figure 4. 5-mm endocanalicular balloon positioned at DCR ostium.
- Powered endoDCR
- Guarded electric burrs for osteotomy: 93% success in 283 cases (Ali, OPRS 2015)
- Can be useful in removing the thicker bone anteriorly
- Stentless endoscopic DCR. Routine use of stents is very common, but there is evidence that they might not be necessary. A number of conflicting studies have been published.
- In a randomized series of 128 eyes, success rates were higher in the stentless group of 65 eyes (89%) versus the stent group of 63 (57%) at 33 months (Mohamed, Ann R Coll Surg Engl 2013).
- Another study demonstrated no difference among 120 randomized patients (Chong, Ophthalmology 2013) and a third demonstrated 91% success among 132 cases without stents at 12 months and no new consecutive canalicular obstruction (Cannon, Ophthalmology 2013).
- A prospective, randomized, controlled interventional trial of 300 patients showed a statistically higher success rate in endonasal DCR surgery with tubes compared with no tubes. Failure rate was more than as twice as high when tubes were not used (12.2% vs. 5.3%) (Fayers, Ophthalmology 2016).
Ongoing controversies
- As endonasal/endoscopic surgical techniques have improved, success rates for endoDCR have also improved, and recent papers report 86%–99% success, compared to 85%–95% for external DCR.
- For ophthalmologists who are less familiar with nasal anatomy and instrumentation, initial endoDCRs might have lower success rates (Onerci, Acta Otolaryngol 2000). For experienced surgeons, OR time and blood loss are similar between the external and endoDCR techniques.
- Some patients and surgeons might prefer the conscious sedation anesthesia that can be used for external DCR but not usually for endonasal DCR (although Tripathi and Magab each report local anesthesia for endonasal DCR; see references).
- However, patients typically prefer the endonasal approach since there is no external scar, no dressing, no bruising, no sutures, and a more rapid return to work (Ozer, Int J Ophthalmol 2014; Gauba, Saudi J Ophthalmol 2014). One published study comparing external to nonlaser endoDCR by a single surgeon reported success rates of 90% and 89%, respectively, and noted that OR time was less for endoDCR (Dolman, Ophthalmology 2003).
- Patients with secondary causes of NLDO may have decreased success rates with endoDCR, in particular those with a history of radioactive iodine therapy and radiotherapy (Sweeney, Ophthalmic Plast Reconstr Surg 2018). Surgeons should discuss decreased success rates of endoDCR (as well as external DCR) with patients with a history of secondary causes of NLDO.
- For the physician as health advocate, there is at least some value in the lack of disturbance to medial canthal anatomy and consequent zero risk of eyelid neurovascular injury from the endonasal approach.
- Finally in cases of acute dacryocystitis for which an external incision might need to be delayed, the endonasal approach can be easily used during the acute infection and serves an important role for drainage of the abscess, shortening the overall course of the disease (Madge, Orbit 2011).
- Nevertheless, among ASOPRS members, external DCR is still preferred (Barmettler, Orbit 2013).
Patient management: treatment and follow-up
- Do not blow nose for 2 weeks to reduce risk of postoperative epistaxis.
- Avoid manipulation of medial canthal area if a stent is placed to avoid prolapse laterally.
- Call surgeon or report to nearest ER for intractable epistaxis.
Medications prescribed:
- Consider topical ophthalmic antibiotic/steroid combination eyedrop for 1–2 weeks. Ointments might clog the outflow system.
- Consider fluticasone or other nasal steroid for 1 month.
- Oral analgesia with acetaminophen or narcotic
Other management considerations:
- Patients are commonly seen 2 weeks postoperatively for office nasal endoscopy and possible irrigation of the operated lacrimal outflow system. The Katena 23gauge bullet-tip cannula is easily fed into a canaliculus alongside the stent (if present); the typical relative ease of delivery into the nasopharynx (compared to preoperatively) is encouraging to the patient.
- Additional office follow-up at 6–8 weeks for nasal endoscopic exam: If the ostium is seen to be healing well without erythema and stenosis, the stent can be removed at 2–3 months or surgeon preference. Mucosal injections of triamcinolone can performed if aggressive fibrosis is seen.
- Some of the early enthusiasm for endoDCR techniques was related to reoperation for failing external DCR; many reports describe successful repeat endoDCR for ostium closure. However for reoperation, consider timing to avoid fibroblast proliferation, and possible use of mitomycin C.
Common treatment responses, follow-up strategies:
- Most patients will have a simple course with relief from epiphora and/or dacryocystitis. Sometimes, epiphora is further reduced upon removal of the silicone stent.
- Biofilm formation on the silicone stent has been described (Parsa, OPRS 2010) and although its clinical significance is not completely understood, it can sometimes cause blepharoconjunctivitis, increased ocular and/or nasal mucus discharge, and inflammation/stenosis of the ostium. Aggressive use of topical steroids and consideration of early removal of the stent (if ostium and/or canalicular inflammation do not abate) is indicated.
- Successful external DCR creates a 2–4mm ostium on average regardless of bony osteotomy size (Bumsted, Arch Otolaryngol 1982; Yazici, Arch Ophthalmol 2003). EndoDCR is likely to have similar results.
- Failing or failed endonasal DCR can occur from mucosal stenosis at the ostium. Repeat endonasal DCR (with or without mitomycin C) can be considered. Balloon dacryoplasty of the ostium for post-surgical stenosis has been reported to have 74% success (Lee, OPRS 2014).
Preventing and managing treatment complications
- Recurrent/progressive dacryostenosis:
- The literature is mixed on the question of ostium size: Size does not predict success (Chan, Ophthalmology 2013) versus a small ostium is associated with failure (Hammoudi, OPRS 2011).
- Associated with failure (Onerci, Acta Otolaryngol 2000):
- Bony spicules left at ostium
- Intranasal synechiae
- Inadequate removal of medial wall of the lacrimal sac
- Consider avoiding heat at the ostium, e.g., laser, Colorado needle (Garcia Vilaro, Ophthalmic Res 2013).
- Ostium granulomas recognized on postoperative endoscopy:
- Treat with intranasal steroid spray, triamcinolone injection, or excision (Ali, Orbit 2015).
- Sump syndrome with chronic mattering and low-grade dacryocystitis:
- Prevent with large uncinectomy and removal of medial wall of lacrimal sac (Fayet, OPRS 2014)
- Additionally it makes sense (although unproven) to prevent sump syndrome by extending the ostium to the inferior end of the lacrimal sac and preventing the flaps from reuniting by excising, suturing, etc.
- Cheese-wiring through puncta/canaliculi:
- Appropriate tension on silicone tubing loop in medial commissure for prevention
- Avoid rigid silicone tubing such as Stent-Tube.
- Consider avoiding silicone intubation altogether (see stentless endoscopic DCR above).
- Perioperative dacryocystitis:
- Consider perioperative antibiotic treatment; not necessarily standard of care.
- Contact dermatitis from topical antibiotic drops:
- Neomycin and others can cause; stop if occurs.
- Intraoperative/postoperative hemorrhage
- Usual precautions regarding aspirin and anticoagulants.
- Multiple packing/nasal tampon options including resorbable nasal materials, Floseal, and nonabsorbable packing.
- Acute Sinusitis
- Typically occurs in the first postoperative week with usual symptoms of facial pain and tenderness and nasal discharge. Responds to oral antibiotics.
- Overall incidence in a retrospective cohort of 203 endoDCR cases was about 1.5%, except for patients with a history of chronic sinusitis, for whom the incidence was 15% (Shams, Eye 2013).
- Intranasal adhesions and synechiae:
- In-office lysis in first postoperative weeks
- Consider therapeutic (or prophylactic) use of resorbable or removable nasal tampon or packing to separate nasal septum and middle turbinate from the lateral nasal wall and ostium
- Cerebrospinal fluid (CSF) rhinorrhea:
- Uncommon; one case with spontaneous resolution on bedrest (Badilla, Arch Ophthalmol 2007)
- Prevent with careful bone removal, avoiding iatrogenic fracture, or removal of bone above the height of the medial canthal tendon.
- Consider early referral to ENT when suspected.
Historical perspective
- External DCR first described by Toti in 1904; previous lacrimal surgery for dacryocystitis described by Galen and Celsus involved using a “red-hot cautery”
- First balloon dacryoplasty described by Hanafee and Dayton in 1978
- Endonasal/endoscopic DCR
- First reported transnasal DCR by Caldwell in 1893; first modern clinical study published in J Laryngol Otol by McDonough and Meiring in 1989.
- In the ophthalmic literature, early reports with Holmium and other lasers were initially published by Massaro, Gonnering, and Harris in 1990.
References and additional resources
- American Society of Ophthalmic Plastic & Reconstructive Surgery, The Tear System. Accessed October 15, 2015.
- AAO, Basic and Clinical Science Course. Section 7: Orbit, Eyelids, and Lacrimal System, 2013-2014.
- AAO, Ophthalmic Technology Assessment: Endonasal Dacryocystorhinostomy, Ophthalmology. 2001:108:2369-2377.
- AAO, Preferred Practice Pattern, Nasolacrimal Duct Obstruction, 2013.
- Ali MJ et al. The Dacryocystorhinostomy Ostium Granulomas: Classification, Indications for Treatment, Management Modalities and Outcomes. Orbit. 2015 Jun;34(3):146-51
- Ali MJ, Psaltis AJ, Murphy J, Wormald PJ. Powered endoscopic dacryocystorhinostomy: a decade of experience. Ophthal Plast Reconstr Surg. 2015; 31(3):219-221.
- Ali MJ, Singh M, Chisty N, Kamal S, Naik MN. Endoscopic ultrasonic dacryocystorhinostomy: clinical profile and outcomes. Eur Arch Otorhinolaryngol. 2016 Jul;273(7):1789-93. doi: 10.1007/s00405-015-3826-z. Epub 2015 Nov 3. PMID: 26530294.
- Badilla J, Dolman PJ. Cerebrospinal fluid leaks complicating orbital or oculoplastic surgery. Arch Ophthalmol. 2007; 125(12):1631-1634.
- Barmettler A, Ehrlich JR, Lelli G Jr. Current preferences and reported success rates in dacryocystorhinostomy amongst ASOPRS members. Orbit. 2013; 32(1):20-6.
- Bumsted RM, Linberg JV, Anderson RL, Barreras R. External dacryocystorhinostomy. A prospective study comparing the size of the operative and healed ostium. Arch Otolaryngol. 1982; 108(7):407-410.
- Camara JG, Bengzon AU, Henson RD. The safety and efficacy of mitomycin C in endonasal endoscopic laser-assisted dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2000;16(2):114-118.
- Cannon PS, Chan W, Selva D. Incidence of canalicular closure with endonasal dacryocystorhinostomy without intubation in primary nasolacrimal duct obstruction. Ophthalmology. 2013; 120(8):1688-1692.
- Chan W, Selva D. Ostium shrinkage after endoscopic dacryocystorhinostomy. Ophthalmology. 2013; 120(8):1693-1696.
- Chong KK, Lai FH, Ho M, Luk A, Wong BW, Young A. Randomized trial on silicone intubation in endoscopic mechanical dacryocystorhinostomy (SEND) for primary nasolacrimal duct obstruction. Ophthalmology. 2013; 120(10):2139-2145.
- Dolman PJ. Comparison of external dacryocystorhinostomy with nonlaser endonasal dacryocystorhinostomy. Ophthalmology. 2003;110(1):78-84.
- Dolmetsch AM. Nonlaser endoscopic endonasal dacryocystorhinostomy with adjunctive mitomycin C in nasolacrimal duct obstruction in adults. Ophthalmology. 2010; 117(5):1037-1040.
- Fayers T, Dolman PJ. Bicanalicular silicone stents in endonasal dacryocystorhinostomy: results of a randomized clinical trial. Ophthalmology. 2016;123(10): 2255-9.
- Fayet B, Katowitz WR, Racy E, Ruban JM, Katowitz JA. Endoscopic dacryocystorhinostomy: the keys to surgical success. Ophthal Plast Reconstr Surg. 2014; 30(1):69-71.
- Fenton S, Cleary PE, Horan E, Murray A, Ho SL, Ryder D, O’Connor G. Balloon dacryocystoplasty study in the management of adult epiphora. Eye (Lond). 2001; 15(Pt 1):67-69.
- Garcia Vilaro M et al. Thermal damage influences endonasal dacryocystorhinostomy success. Ophthalmic Res. 2013; 49(4):209-214.
- Gauba V. External versus endonasal dacryocytorhinostomy in a specialized lacrimal surgery center. Saudi J Ophthalmol. 2014; 28(1):36-9.
- Gustafson RO, Bartley GB. A plea for preservation of the middle turbinate during dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 1999; 15(2):75-76.
- Hammoudi DS, Tucker NA. Factors associated with outcome of endonasal dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2011; 27(4):266-269.
- Hodgson N, Bratton E, Whipple K, Priel A, Oh SR, Fante RG, Kikkawa DO, Korn BS. Outcomes of endonasal dacryocystorhinostomy without mucosal flap preservation. Ophthal Plast Reconstr Surg. 2014; 30(1):24-27.
- Jang SY, Lee KH, Lee SY, Yoon JS. Effects of nasopore packing on dacryocystorhinostomy. Korean J Ophthalmol. 2013; 27(2):73-80.
- Jutley G et al. Patient satisfaction following endoscopic endonasal dacryocystorhinostomy: a quality of life study. Eye (Lond). 2013; 27(9):1084-1089.
- Kamal S, Ali MJ, Naik MN. Circumostial injection of mitomycin C (COS-MMC) in external and endoscopic dacryocystorhinostomy: efficacy, safety profile, and outcomes. Ophthal Plast Reconstr Surg. 2014; 30(2):187-190.
- Kao SC, Liao CL, Tseng JH, Chen MS, Hou PK. Dacryocystorhinostomy with intraoperative mitomycin C. Ophthalmology. 1997; 104(1):86-91.
- Kuchar A, Steinkogler FJ. Antegrade balloon dilatation of nasolacrimal duct obstruction in adults. Br J Ophthalmol. 2001;85(2):200-204.
- Lanciego C, Toledano N, De Miguel S, Perea M, Padilla M, Rodriguez-Merlo R, Dávila J, Ibarburen C, Cano C, García IR, García LG. Resolution of epiphora with nasolacrimal stents: results of long-term follow-up in a multicenter prospective study. J Vasc Interv Radiol. 2003; 14(11):1417-1425.
- Lee A, Ali MJ, Li EY, Wong AC, Yuen HK. Balloon dacryoplasty in internal ostium stenosis after endoscopic dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2014; 30(1):7-10.
- Madge SN, Chan W, Malhotra R, Ghabrial R, Floreani S, Wormald PJ, Tsirbas A, Selva D. Endoscopic dacryocystorhinostomy in acute dacryocystitis: a multicenter case series. Orbit. 2011; 30(1):1-6.
- Maini S, Raghava N, Youngs R, Evans K, Trivedi S, Foy C, Mackintosh G. Endoscopic endonasal laser versus endonasal surgical dacryocystorhinostomy for epiphora due to nasolacrimal duct obstruction: prospective, randomised, controlled trial. J Laryngol Otol. 2007; 121(12):1170-1176.
- Marcet MM, Kuk AK, Phelps PO. Evidence-based review of surgical practices in endoscopic endonasal dacryocystorhinostomy for primary acquired nasolacrimal duct obstruction and other new indications. Curr Opin Ophthalmol. 2014; 25(5):443-8.
- McCullough KM. Naso-lacrimal duct balloon dilatation: medium to long term follow-up. Clin Radiol. 2001; 56(1):13-16.
- Mohamed SH, Khan I, Shakeel M, Nandapalan V. Long-term results of endonasal dacryocystorhinostomy with and without stenting. Ann R Coll Surg Engl. 2013; 95(3):196-199.
- Moore WM, Bentley CR, Olver JM. Functional and anatomic results after two types of endoscopic endonasal dacryocystorhinostomy: surgical and holmium laser. Ophthalmology. 2002; 109(8):1575-1582.
- Nagi KS, Meyer DR. Utilization patterns for diagnostic imaging in the evaluation of epiphora due to lacrimal obstruction: a national survey. Ophthal Plast Reconstr Surg. 2010; 26(3):168-171.
- O’Donnell B, Shah R. Dacryocystorhinostomy for epiphora in the presence of a patent lacrimal system. Clin Experiment Ophthalmol. 2001; 29(1):27-29.
- O’Donnell B, Clement CI. Assessing patients with epiphora who are patent to syringing: clinical predictors of response to dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2007; 23(3):173-178.
- Onerci M, Orhan M, Ogretmenoğlu O, Irkeç M. Long-term results and reasons for failure of intranasal endoscopic dacryocystorhinostomy. Acta Otolaryngol. 2000; 120(2):319-322.
- Ozer S, Ozer PA. Endoscopic vs external dacryocystorhinostomy-comparison from the patients’ aspect. Int J Ophthalmol. 2014; 7(4):689-696.
- Parsa K, Schaudinn C, Gorur A, Sedghizadeh PP, Johnson T, Tse DT, Costerton JW. Demonstration of bacterial biofilms in culture-negative silicone stent and jones tube. Ophthal Plast Reconstr Surg. 2010; 26(6):426-430.
- Patel V, Ross JJ, Malhotra R. Early experience using a new modified bone nibbler for superior osteotomy during endonasal dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2011; 27(1):15-20.
- Ragab SM, el-Koddousy MS, Badr M. Endocanalicular, high-pressure balloon catheter, endoscopic dacryocystorhinostomy: a randomized controlled trial. Otolaryngol Head Neck Surg. 2011; 145(4):683-688.
- Shams PN et al. Acute post-operative rhinosinusitis following endonasal dacryocystorhinostomy. Eye (Lond). 2013; 27(10):1130-1136.
- Shams PN, Wormald PJ, Selva D. Anatomical landmarks of the lateral nasal wall: implications for endonasal lacrimal surgery. Curr Opin Ophthalmol. 2015; 26(5):408-15.
- Silbert DI, Matta NS. Outcomes of 9 mm balloon-assisted endoscopic dacryocystorhinostomy: retrospective review of 97 cases. Orbit. 2010; 29(3):131-134.
- Sweeney AR, Davis GE, Chang SH, Amadi AJ. Outcomes of endoscopic dacryocystorhinostomy in secondary acquired nasolacrimal duct obstruction: a case-control study. Ophthalmic Plast Reconstr Surg. 2018; 34(1):20-25.
- Tripathi A, Lesser TH, O’Donnell NP, White S. Local anaesthetic endonasal endoscopic laser dacryocystorhinostomy: analysis of patients’ acceptability and various factors affecting the success of this procedure. Eye (Lond). 2002; 16(2):146-149.
- Trotter WL, Meyer DR. Endoscopic conjunctivodacryocystorhinostomy with Jones tube placement. Ophthalmology. 2000; 107(6):1206-1209.
- Tsai CC et al. Efficacy of probing the nasolacrimal duct with adjunctive Mitomycin-C for epiphora in adults. Ophthalmology. 2002; 109(1):172-174.
- Ugurbas SH, Zilelioglu G, Sargon MF, Anadolu Y, Akiner M, Aktürk T. Histopathologic effects of mitomycin-C on endoscopic transnasal dacryocystorhinostomy. Ophthalmic Surg Lasers. 1997; 28(4):300-304.
- Velegrakis GA, Prokopakis EP, Panayotaki I, Pagalos AG, Siganos CS, Helidonis ES. Intranasal laser-assisted dacryocystorhinostomy with the use of a surgical microscope. Am J Otolaryngol. 2002; 23(5):272-276.
- Watkins LM, Janfaza P, Rubin PA. The evolution of endonasal dacryocystorhinostomy. Surv Ophthalmol. 2003;48:73-84. Comment in: Surv Ophthalmol. 2004;49:543; author reply 544.
- Weiss M, Lauer SA, Fried MP, Uribe J, Sadoughi B. Endoscopic endonasal surgery simulator as a training tool for ophthalmology residents. Ophthal Plast Reconstr Surg. 2008; 24(6):460-464.
- Xue K, Mellington FE, Norris JH. Meta-analysis of the adjunctive use of mitomycin C in primary and revision, external and endonasal dacryocystorhinostomy. Orbit. 2014; 33(4):239-244.
- Yazici B, Yazici Z. Final nasolacrimal ostium after external dacryocystorhinostomy. Arch Ophthalmol. 2003; 121(1):76-80.
- Ziahosseini K, Al-Abbadi Z, Malhotra R. Botulinum toxin injection for the treatment of epiphora in lacrimal outflow obstruction. Eye. 2015; 29(5):656-61.
