Enucleation and Evisceration
Updated May 2024
Goals of procedure
Enucleation
- Alleviation of pain from a blind painful eye
- Decrease risk of sympathetic ophthalmia after traumatic globe perforation
- Removal of intraocular tumor to prevent tumor spread
- Primary infection prevention in a nonrepairable globe rupture
- Restore orbital volume
- Cosmesis for disfigured eye
Evisceration
- Alleviation of pain from a blind painful eye
- Restore orbital volume
- Cosmesis
- Infection resolution in refractory endophthalmitis
Indications and contraindications
Enucleation
- Indications
- Primary intraocular malignancies not amenable to alternative treatment
- Selected cases of ocular trauma with loss of visual potential
- 66% of traumatic enucleations were in children 16 years or younger (Am J Ophthalmol 1992; 113:138).
- Risk factors for post-traumatic enucleation include
- Poor initial visual acuity (light perception or no light perception)
- Wound length greater than 10 mm
- Being a young male (Ophthalmology 1995; 102:393)
- Open globe injuries from blunt trauma are more likely to require enucleation than those caused by sharp objects.
- Endophthalmitis
- Blind painful eye
- Severe infection, e.g., corneal ulceration, perforation, and panophthalmitis
- Contraindications
- Relative
- Eye with visual potential
- Intraocular tumor with metastatic disease
- Extraocular tumor extension
- Absolute
- None
Evisceration
- Indications
- Blind painful eye in which intraocular malignancy has been excluded
- Endophthalmitis
- Might be preferable to enucleation in patients in whom
- General anesthesia is contraindicated and/or shorter, technically simpler procedure preferred
- Bleeding diathesis is present
- Maximal cosmesis is a priority
- Conjunctival scarring is present to decrease risk of further socket contracture
- Contraindications
- Relative
- Theoretic concern for risk of sympathetic ophthalmia (the validity of increased risk of SO after evisceration, as compared to enucleation, has been countered by multiple studies) (Manandhar, Nepal J Ophthalmol, 2011)
- Severe phthisis bulbi
- Absolute
- Intraocular malignancy
Preprocedure evaluation
Complete ophthalmic and medical histories
- Previous ophthalmic surgery, i.e., conjunctival scar, scleral buckle, glaucoma implants
- Cancer
- Trauma
- Acetylsalicylic acid (ASA)/antiplatelet/anticoagulant/anti-inflammatory therapy
Clinical examination
- Comprehensive eye examination including
- Careful visual acuity assessment
- Pupil exam
- Dilated funduscopic exam
- Assessment of globe volume/proptosis
- In relation to contralateral eye
- Any increase in orbital volume, e.g., fracture
- In cases of trauma
- Extent of corneoscleral laceration
- Presence of prolapsed uvea
- Systemic examination by primary doctor or internist if systemic disease or metastatic tumor is suspected
- Possible second opinion to assess for visual potential
Preoperative assessment
- B-scan ultrasonography if media opaque to help rule out intraocular malignancy
- Other additional imaging options (on case-by-case basis)
- Orbit computed tomography (CT)
- Magnetic resonance imaging (MRI) scan
Alternatives to procedure
- Observation with medical therapy (for blind painful eye without neoplasm)
- Atropine and prednisolone ophthalmic drops
- Retrobulbar alcohol injection
- Retrobulbar chlorpromazine
- Observation with medical therapy may not be uniformly effective in reducing pain and may require serial injections or drop administrations
- Other management options can be considered for individual patients on a case-by-case basis:
- Evisceration and enucleation can often be considered as alternatives for each other with the important exception of intraocular tumor, in which case evisceration is contraindicated.
- Fitting of a cosmetic scleral shell prosthesis
Enucleation anesthesia, instrumentation, and technique
Anesthesia options
- Monitored anesthesia care (MAC) anesthesia: retrobulbar anesthesia with sedation
- General anesthesia
- Consider retrobulbar bupivacaine and 2% lidocaine with epinephrine 1:100,000 injection prior to and at end of the procedure for local pain control and hemostasis.
Instrumentation
- 0.5 forceps and/or Bishop-Harmon forceps
- Muscle hook
- Castroviejo needle driver
- Bipolar cautery
- 11 blade
- Kelly or 90-degree clamp
- Mosquito hemostat or bulldog/Serrafine clips
- Westcott and Stevens’ tenotomy scissors
- Metzenbaum or enucleation scissors
- Sponges and cotton tipped applicators
- Thrombin, local anesthetic, or cocaine: can soak sponges and apply for local hemostasis
- Malleable retractor
- Implant (see below)
- Suture
- 6-0 plain gut
- 5-0 or 6-0 polyglactin
- 4-0 silk
Technique
(Jordan, Int Ophthalmol Clin 2006; Moshfeghi, Surv Ophthalmol 2000)
- 360-degree conjunctival limbal peritomy using Westcott scissors
- Dissection of Tenon layer away from the globe in each of the four quadrants
- Each rectus muscle is isolated with muscle hooks and any remaining adhesions are cleaned away with a cotton-tipped applicator.
- 5-0 or 6-0 polyglactin suture is placed just posterior to the muscle insertion to secure it.
- The insertion can then be severed from the globe with Westcott scissors.
- It can be helpful to leave a 2–3-mm stump of tendon that can be grasped with forceps or secured with suture to help manipulate the globe later in the procedure.
- The oblique muscles are identified and released form the globe.
- These can be left to retract in the orbit, although some surgeons might tag them for later attachment to the implant.
- A 4-0 silk traction suture can be attached to the lateral rectus insertion, which can be used to prolapse the globe out of the orbit to gain better access to the optic nerve and increase the length of nerve obtained.
- A helpful technique to identify the optic nerve is to “strum” it with the enucleation or curved Metzenbaum scissors, then gently slide the blades of the scissors around the nerve and transect it as close to the apex of the orbit as possible.
- Pressure is applied to the socket with an orbital implant sizer wrapped in gauze or thrombin- or cocaine-soaked gauze to achieve hemostasis.
- If there is persistent bleeding, malleable retractors can be used to retract orbital fat away from the optic nerve stump and allow direct visualization of active bleeding vessels for cauterization.
- At least 1 cm of optic nerve should be obtained.
- Submit the globe for histological analysis.
- No perforations should be made into the globe for the purpose of formalin fixation.
- Time alone will allow for penetration of formalin through the intact globe.
- Orbital implant placement (see below)
- Attachment of EOM to implant, implant wrapping or the fornix using the preplaced polyglactin sutures in a position analogous to their physiologic positions on the globe.
- Anterior Tenon capsule is closed over the implant with buried 4-0 or 5-0 polyglactin suture.
- Conjunctival closure without tension using a 6-0 plain gut suture; a rapid absorbing plain gut suture is not recommended.
- Care should be taken to prevent “burying” conjunctival edges because conjunctival cysts can form later.
- Place a conformer of appropriate size to prevent socket contracture. Conformers can be left in for 4–6 weeks until prosthesis fitting (Ophthal Plast Reconstr Surg 1998; 14:144).
- Antibiotic/steroid ointment can be applied to the socket daily.
- Possible temporary intermarginal suture placement
- Pressure patch placement
- Can rarely consider lateral orbitotomy to increase exposure in select cases, e.g., retinoblastoma, in which a long segment of optic nerve is required to increase the chances of complete resection.
- Consideration for orbital implant peg or other coupling device
- A peg placed in the implant can be coupled to an ocular prosthesis to allow a more natural appearing and moving artificial eye
- A pegged prosthesis requires special care and regular follow up visits
- A properly selected patient can make a good candidate for pegging. Consider pegging in a healthy adult who is responsible and capable of looking after the artificial eye and returning for regular follow-up visits.
- Peg placement should be considered only after fibrovascularization of the implant has been completed, generally at 5–6 months after implant placement.
- Fibrovascularization can be confirmed with gadolinium-enhanced MRI. Drilling into an avascular area of the implant might predispose to infection.
- Technique for pegging (Jordan, Int Ophthalmol Clin 2006):
- Preoperatively, with the patient sitting upright, mark the center of the implant with a marking pen. This process can be facilitated with the use of a conformer custom-made by the ocularist with a hole in the center to align the mark.
- Local anesthesia is infiltrated into the upper and lower eyelids. A retrobulbar injection should not be used as it can alter the orbital volume and ocular motility which need to be assessed for accurate drilling.
- With the patient in a supine position, a 1.5-inch 25-gauge needle is used to make the initial guide hole to a depth of 5 mm. The patient is sat upright and asked to look in all fields of gaze to assess alignment. Adjustments are easily made to the needle position.
- Once correctly aligned, the needle is removed and an 18-gauge needle is used to perform the same maneuver. Once the proper alignment is confirmed, it is advanced to a depth of 10 mm.
- The 18-gauge needle is replaced with a handheld drill bit and it is slowly rotated and advanced into the implant along the tract created by the 18-gauge needle. The depth of the hole can be measured with the wooden end of a cotton-tipped applicator. A retrobulbar injection can now be given for pain control.
- The drill hole is irrigated with an antibiotic solution. The sleeve shaft is carefully screwed into position until it fits tight and flush with the implant surface.
- A temporary flat-headed peg is slipped into position and Tenon layer closed with buried 6-0 polyglactin suture. Conjunctiva is closed with plain gut suture.
- The temporary flat-headed peg is exchanged for the permanent round-headed peg by the ocularist at 4–5 weeks.
- Polycarbonate pegs have largely been supplanted by titanium pegs due to their better biocompatibility and reduced complication rate.
Implants and technique of implant placement
- Orbital implants are necessary after enucleation in order to restore volume, maximize motility of the prosthesis and enhance cosmesis by preventing superior sulcus deformity and enophthalmos.
- Although the type of implant appears to play a role in exposure rates, studies have shown no significant effect of age, sex, ocular diagnosis, surgeon, implant size, chemotherapy, or radiotherapy on exposure rates (Ophthalmology 2000; 107:940, Am J Ophthalmol 2004; 138:425).
- A 2004 survey of ASOPRS members found that porous polyethylene was used in 42.7% of cases and hydroxyapatite was used in 27.3% (Ophthal Plast Reconstr Surg 2004; 20:274).
- Implants can be placed fully in the intraconal space or partly intraconal and partly within Tenon’s space.
- The implant can be inserted with an implant introducer to avoid dragging anterior Tenon fascia posteriorly with the implant.
- Porous/integrated implants have the main advantages of allowing fibrovascular ingrowth and attachment of extraocular muscles for presumed improved motility.
- The fully vascularized implant is better anchored in the orbit than nonintegrated implants perhaps leading to less extrusion and infection.
- If infected, the integrated implant may be successfully treated with systemic antibiotics due to its vascularity (Rosen, Plast Reconstr Surg 1991).
- Coralline hydroxyapatite (HA)
- FDA approved in 1989
- Biocompatible
- Interconnecting pores allow host fibrovascular ingrowth which can reduce the risk of migration, extrusion, and infection
- Minimal foreign body reaction
- Secure attachment of extraocular muscles to implant to improve motility
- Can integrate pegs to allow coupling of a prosthesis for improved motility
- Might require implant wrap
- Implant exposure appears to be related to surgical technique rather than the implant material
- Some surgeons suggest that calcific HA implants should be avoided in children with retinoblastoma as iatrogenically introduced calcium can confuse evaluation for recurrence of this tumor and complicate secondary irradiation of the orbit, but their use has been described (Shields, OPRS 2015).
- Synthetic HA
- Identical chemical composition to HA
- Smaller and less uniform pore size
- Can be drilled by hand without motorized high-speed drills
- Similar side effect profile to HA
- Less expensive than HA
- Porous polyethylene (PP) is today one of the most widely used orbital implants. A higher incidence of exposure has been reported in children after enucleation for RB than in adults. This complication is thought to be due to the rough surface of PP that can erode the overlying Tenon capsule and conjunctiva.
- Large pore size for greater fibrovascular ingrowth
- Smoother surface than HA for easier implantation and less irritation of overlying conjunctiva
- Can be used with or without wrapping material
- Smooth surface tunnel PP (SSTPP) is a variation on PP with a smooth anterior surface and has been shown to have a low exposure rate (Choi, Br J Ophthalmol 2013).
- Aluminum oxide (Al2O3) ceramic implant
- Human fibroblasts and osteoblasts proliferate more rapidly on Al2O3, which can be more biocompatible than HA (Christel, Clin Orthop 1992; Mawn, Can J Ophthalmol 2001).
- Smoother surface than HA might make it less likely to extrude through the overlying tissues and cause less postoperative inflammation.
- Has similar side effect profile to HA, although they appear to occur less commonly (Jordan, Ophthal Plast Reconstr Surg 2003)
- Nonintegrated implants do not allow fibrovascular ingrowth or extraocular muscle attachment. Their main purpose is volume replacement. However, muscles can be imbricated over the implant to help prevent extrusion and provide some motility. Some believe these options are inferior to using an integrated sphere (Jordan, Int Ophthalmol Clin 2006).
- Acrylic
- Silicone
- PMMA
- Wrapping material can be used to cover rough-surfaced implants such as HA as well as to provide a site to anchor EOM. These smooth materials make insertion easier and provide improved volume augmentation and motility while decreasing rates of extrusion. In a survey of ophthalmic plastic surgeons, however, the majority (59%) preferred not to wrap as the extra time, cost and work were felt to outweigh the benefits (Su, Ophthal Plast Reconstr Surg 2003).
- Donor sclera
- Wrapped around implant and secured with 4-0 or 5-0 nonabsorbable suture
- Theoretical risk of transmissible disease, e.g., prions
- No cases of HIV transmission have ever been reported
- Expensive
- Autologous tissue offers the advantage over donor sclera of quick vascularization and no foreign body response. If not using autologous sclera from the enucleated eye, disadvantages include need for a second surgical site for harvest and the associated surgical risks and scarring.
- Sclera: It is important to note that autologous sclera is not appropriate if it is harvested from an eye with a tumor, e.g., RB.
- Temporalis fascia
- Fascia lata
- Dermis
- Pericardium
- Auricular muscle complex
- Pericranium
- Synthetic mesh has no risk of disease transmission or harvest requirement.
- Vicryl
- Polytetrafluoroethylene (PTFE, Gore-Tex)
- Expanding implants are an important consideration in children. Although the globe has been measured to have a volume of 7 mL, a final expander size of 5 mL is generally the target. When it is coupled with the eye prosthesis, these combined give the final volume of 7 mL (Gundlach, Plast Reconstr Surg 2005).
- Serial conformer fittings
- Serially enlarged spherical implants
- Dermis-fat grafts (Smith, Ophthalmology 1982)
- Studies have shown that in children under the age of 4 years, the dermis-fat graft can grow, thus stimulating continued orbital expansion as the child grows (Heher, Ophthal Plast Reconstr Surg 1998; Mitchell, J AAPOS 2001).
- The dermal component provides structural support for ingrowth of conjunctiva and vascularization, minimizing reabsorption
- More resistant to reabsorption than free fat grafts (Leaf, Arch Surg 1972)
- Self-inflating hydrogel expanders
- Classically described for management of congenital anophthalmia or microphthalmos
- Spheres and pellets are ideal for expansion of bony orbit
- Hemispherical expanders are ideal for implantation on the conjunctival surface to widen the palpebral aperture
- Made of highly hydrophilic N-vinylpyrrolidone and methylmethacrylate
- Attracts water from surrounding tissues by osmosis
- Amount and rate of expansion can be precisely engineered by manufacturer
- The size of the volume deficit can be measured by placing a prosthesis then injecting sterile saline solution or local anesthesia into the muscle cone with a retrobulbar needle until symmetry with the contralateral side is achieved
- Expansion can be noted at POD 1 and maximum volume is attained at 1 week (Hou, J AAPOS 2012).
- Orbital volume has been reported to increase from 75% to 84% of the contralateral unaffected orbit and the horizontal palpebral length from 71% to 85.4% of the contralateral unaffected eye in patients treated for microphthalmos (Hou, J AAPOS 2012). In a study of patients with anophthalmia, orbital volume increased from 46% to 70% compared to the contralateral side and palpebral fissure length increased from 60% to 73% compared to the contralateral side (Gundlach, Plast Reconstr Surg 2005).
- Inflatable saline expanders (Dunaway, Br J Plast Surg 1996)
- Shown to enlarge the orbit with establishment of near normal bony growth patterns
- Has a less dramatic effect on growth of eyelids and eyelid fissure
- Technique for expanding implant placement
- Dermis-fat graft
- The enucleation proceeds as described in the Enucleation in Adults section including attachment of the free end of the extraocular muscles to a double-armed 5-0 polyglactin suture.
- The donor site is selected in a nonhair-bearing area, e.g., upper outer quadrant of the buttock.
- An adequately sized graft is critical with an anterior lamellar (dermis) diameter between 18 and 25 mm and a depth of 25 mm.
- The donor site is measured and marked, then outlined with a 10 Bard Parker blade. The skin is dermabraded with a hand-held wire brush, scalpel blade, scissors, or high-speed drill with diamond burr.
- A deep incision is made perpendicular to the surface through dermis and subcutaneous fat without beveling the incision. The dermis-capped fat is severed from the donor.
- The graft is then placed within the extraocular muscle cone. The preplaced polyglactin sutures of each extraocular muscle (see enucleation technique above) are then passed through the dermal margin of the graft then through Tenon fascia and finally conjunctiva, starting with the superior rectus and proceeding in a clockwise fashion. Any excess, prolapsing fat should be reposited into the orbit, or it can be trimmed if this is not possible.
- Interrupted 5-0 chromic sutures should be used to secure the remaining Tenon fascia and conjunctiva to the dermis between the extraocular muscle polyglactin sutures.
- A fornix conformer is placed and interpalpebral sutures are tied temporarily.
- The donor wound is closed with 3-0 or 4-0 absorbable sutures in a buried, interrupted fashion, and 4-0 or 5-0 superficial sutures in a running, mattress, or other fashion.
- Spherical expander
- Come in sizes ranging from 1 to 5 mL
- The expander is placed in the intraconal space followed by imbrication of the extraocular muscles and closure of the conjunctiva.
- A prosthesis can be placed at this time, followed by temporary tarsorrhaphy for 1 week (Hou, J AAPOS 2012).
- Injectable pellet expander
- Each pellet expands to a final volume of 0.24 mL.
- The number of pellets can be calculated by taking the volume needed to be replaced in mL (measured as described above) and dividing by 0.24 mL.
- Positioned in the orbit by injection with a customized trocar using a retrobulbar injection technique into the intraconal space (Hou, J AAPOS 2012)
- Inflatable saline expanders
- Classically described for management of congenital anophthalmia or microphthalmos, but can be performed after enucleation for microphthalmos
- A bicoronal scalp flap of periosteum is raised from the superior, lateral and inferior walls of the orbit
- An incision is made in the reflected orbital periosteum superiorly and a 4-mL spherical tissue expander is placed within the orbital soft tissues
- The filling port tube is passed through a further small incision placed posteriorly and laterally in the orbital periosteum. The tube then passes through a small hole in the lateral orbital rim into the temporal fossa and up onto the scalp. Injection ports are placed subcutaneously in the temporoparietal region.
- A tarsorrhaphy is placed and 2 mL of saline are injected into the expander.
- In patients more than 2 years old, osteotomies are placed through the frontozygomatic suture, lateral orbital wall, zygomatic bone and arch, lateral part of the orbital floor and inferior orbital rim
- Inflation of the expander starts 2 weeks postimplantation and continues are varying rates that are individualized to each patient.
Evisceration anesthesia, instrumentation, and technique
Anesthesia options
- Same as enucleation
Instrumentation
- 0.5 forceps and/or Bishop-Harmon forceps
- Muscle hook
- Castroviejo needle driver
- Bipolar cautery
- 11 blade
- Mosquito hemostat or bulldog/Serrafine clips
- Wescott and Stevens’ tenotomy scissors
- Sponges and cotton tipped applicators
- Thrombin, local anesthetic, or cocaine: can soak sponges and apply for local hemostasis
- Evisceration spoon
- Implant (see section on enucleation)
- Suture
- 6-0 plain gut
- 5-0 or 6-0 polyglactin
- 4-0 silk
Technique
(Nerad, Oculoplastic Surgery, The Requisites. St. Louis: Mosby, 2001)
- Evisceration can be performed with or without removal of the cornea.
- If the cornea is healthy, it can be preserved:
- Conjunctival periotomy with Wescott scissors, dissecting only a few millimeters posterior to the limbus
- The superior rectus is tagged and disinserted from the globe as described for enucleation
- An incision through the sclera is made posterior to the superior rectus insertion
- The intraocular contents are removed
- An implant is placed (see implants section above)
- Scleral wound is closed
- Superior rectus is reattached to its insertion
- If the cornea will not be preserved:
- Conjunctival peritomy with Wescott scissors, dissecting only a few millimeters posterior to the limbus
- Initiate a keratectomy by making a stab incision through the cornea at the limbus
- Complete the keratectomy by making a full-thickness, 360-degree limbal incision through the cornea and remove it with toothed forceps.
- Remove the intraocular contents with an evisceration spoon by inserting the spoon between the choroid and sclera to deliver the intraocular contents
- The interior of the scleral shell can be scraped with a blade and scrubbed with 100% alcohol to remove all visible uveal tissue remnants (Huang, Am J Ophthalmol 2009)
- Posterior scleral relaxing incisions are usually required to place a 20–22-mm implant, without tension on the imbricated scleral closure
- Place an implant (see implants section above)
- Relaxing incision might be necessary to fit the implant through the anterior opening in the sclera
- Close the wound in 3 layers:
- Overlap the edges of the sclera and close with sutures, e.g., 5-0 polyester or polyglactin in a horizontal mattress fashion
- Pull the edges of Tenon’s capsule together and close with interrupted 5-0 polyglactin suture
- Close the conjunctiva with a running absorbable suture, e.g., plain gut or polyglactin
- Place topical antibiotic and conformer in the conjunctival fornix
- Consider temporary suture tarsorrhaphy
- Consider pressure patch
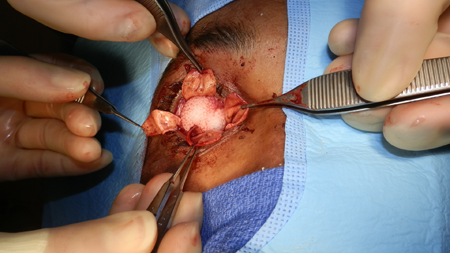
Figure 1. Evisceration. Courtesy Brett Davies, MD.
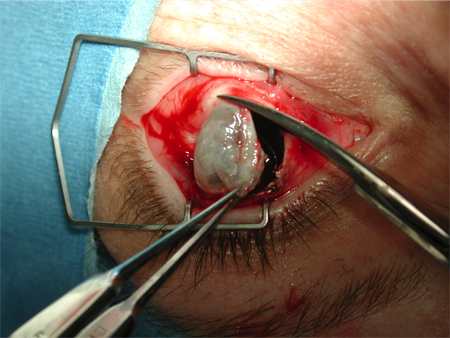
Figure 2. Evisceration. Courtesy Rona Z. Silkiss, MD, FACS.
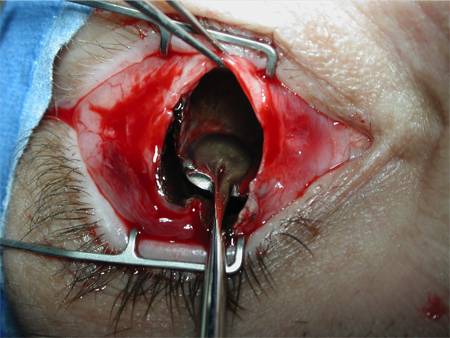
Figure 3. Evisceration. Courtesy Rona Z. Silkiss, MD, FACS.
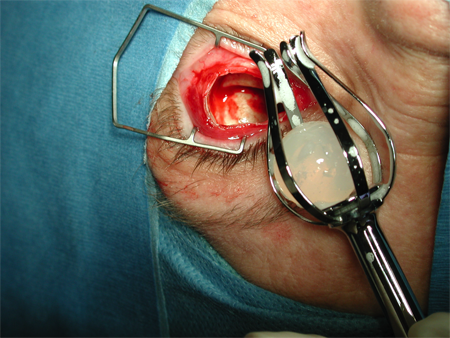
Figure 4. Evisceration. Courtesy Rona Z. Silkiss, MD, FACS.
Patient management: treatment and follow-up
- Postoperative instructions
- Daily antibiotic ointment after patch removed
- Tarsorrhaphy suture removal 1 week after surgery if this was used
- Consider tight patch for 5–7 days (patient need not apply ointment if this patch is used)
- Take oral pain medication as needed
- Avoid strenuous activity during the first week
- Prosthesis fitting with ocularist 4-6 weeks after surgery
- Polycarbonate lens/safety frame prescription for fellow eye at all times
- Prosthesis hygiene
- Topical lubricants
- Periodic prosthesis inspection/polishing/replacement at ocularist
Preventing and managing treatment complications
Intraoperative complications
(Moshfeghi, Surv Ophthalmol 2000)
- Prevention: removal of wrong eye
- Confirm operative site by discussion with patient in preoperative area, marking of skin around eye, examination of eye, review of chart, and ophthalmoscopic examination of eye in operating room (OR), if appropriate.
- Adhere to the operating room “time-out” protocol.
- Prevention: evisceration of an eye with unsuspected intraocular tumor
- In eyes with no view of the posterior pole, optimal imaging studies should be performed to rule out unsuspected neoplasia.
- No imaging is perfect, and small tumors can go undetected.
- Prevention: intraoperative hemorrhage:
- Ask patients to stop anticoagulants in the perioperative period if appropriate.
- Retrobulbar anesthetic with epinephrine can reduce hemorrhage, as can clamping the optic nerve with a hemostat for several minutes before transection.
- If hemorrhage does occur, it can be controlled with packing and pressure applied within the muscle cone or with application of gelfoam, cocaine, or thrombin. Identifiable bleeding vessels can be cauterized.
- Prevention: EOM injury or loss
- Careful placement of traction sutures
- If a muscle is lost, it can be easily located by grasping Tenon’s fascia with forceps in the meridian of the muscle in a hand-over-hand fashion until the muscle is found.
- In fact, a surgical variation is to cut the muscles off the globe without tagging and locating them in the orbit after the eye is removed.
- Temporary removal of implant can facilitate muscle recovery.
- Levator palpebrae superioris injury
Postoperative complications and management
- Early complications
- Orbital hemorrhage and edema
- Prevention: compressive pressure bandage for 3–5 days postenucleation
- Management: A large hematoma might require surgical exploration and drainage.
- Orbital infection
- Prevention
- Meticulous surgical technique
- Topical and systemic antibiotics are used prophylactically in some centers, but no evidence that infection rates are reduced (Fay, OPRS 2013).
- Management
- Topical and systemic antibiotics
- Removal of implant and drainage of any abscess
- Conformer extrusion can lead to conjunctival prolapse with scarring and forniceal shortening.
- Prevention and management is with a temporary intermarginal tarsorrhaphy.
- Late complications
- Deepened superior sulcus is caused by loss of orbital volume either from inadequate replacement or orbital fat atrophy and relaxation of tissues within the orbit. Clinically, it appears as a deep groove between the upper eyelid and orbital rim with enophthalmos and ptosis. Numerous techniques can be used to manage this complication including:
- Methylmethacrylate subperiosteal implant (Spivey, Am J Ophthalmol 1976)
- Suturing the levator complex tendon to the periosteum of the superior orbital rim (Riebel, Br J Ophthalmol 1976)
- Dermis fat grafting of the superior lid (Smith, Ophthalmology 1983)
- Injectable filler materials can be useful to augment orbital volume (Malhotra Arch Ophthal 2007, Olver OPRS 2010).
- Fornix contracture can occur due to shortening of the posterior lamella, implant extrusion, or trauma and can include complete obliteration of the fornices with inability to retain the prosthesis.
- Prevention
- Preservation of conjunctiva during surgery
- Limited conjunctival dissection
- Conformer use
- Management
- Prosthesis modification
- Topical anti-inflammatory therapy
- Fornix/socket reconstruction with mucous membrane/amniotic membrane grafts
- Orbital implant exposure/extrusion
- Etiology
- Implant size, shape, and material might increase risk of exposure/extrusion (“bumpy” or irregular surface creates pressure points and might increase exposure rate)
- Anterior placement of implant
- Inadequate Tenon’s capsule closure
- Closure of conjunctiva under tension
- Poor wound healing
- Infection
- Poor conformer/prosthesis fit
- Foreign body inflammatory response to implant material
- Management
- Observation/topical antibiotics/spontaneous closure (small defects)
- Tissue grafts placement (donor sclera, allogenic dermis, autologous grafts, hard palate, dermis fat, temporalis fascia, tarsoconjunctival grafts, or flaps)
- Implant removal/replacement with reconstruction via possible graft placement as either a single-stage or delayed procedure
- Ectropion
- Due to increased lid laxity, skin cicatrix formation
- Management
- Medial +/- lateral canthal tendon tightening
- Skin graft placement
- Entropion
- Due to socket/fornix contracture
- Management
- Prosthesis modification
- Marginal rotation surgery
- Fornix/socket reconstruction, mucous membrane grafting
- Ptosis
- Etiology
- Damage to the levator muscle or nerve supply
- Levator aponeurosis disinsertion
- Superotemporal implant migration
- Superior fornix scarring
- Management
- Prosthesis modification
- Levator surgery
- Frontalis suspension
- Poor prosthesis motility
- Etiology
- Lost, restricted or paretic muscles
- Inadequate conjunctiva
- Poor prosthesis fit
- Inadequate implant volume or implant migration
- Management
- Reattachment of muscles
- Implant exchange or orbital volume enhancement
- Conjunctival augmentation or restore fornices
- New prosthesis
- Peg placement
- Giant papillary conjunctivitis-chronic mucous discharge
- Prosthesis hygiene
- Topical corticosteroids, nonsteroidal anti-inflammatory agents and mast cell stabilizers
- Lubrication
- New prosthesis if other medical measures fail
- Sympathetic ophthalmia after evisceration
- Extremely low risk after evisceration
- Managed with immunomodulatory therapy
- Complete removal of inciting eye might not be helpful.
- Complications of expandable implants
- Dermis-fat graft
- Intraoperative
- Same as nonexpandable implants
- Donor site complications include hemorrhage and hematoma.
- Postoperative
- Inadequately sized grafts supply inadequate volume.
- Oversized grafts risk devitalization.
- Prevention and Management
- Meticulous technique
- Careful graft size measurements
- Expandable implant
- Intraoperative
- Same as nonexpandable implants
- Postoperative
- Erosions, fibrosis, granuloma formation, migration, and extrusion
- Failures have been reported mostly due to poor surgery or wrong implant shape or size, and can have an incidence of 28% under these circumstances (Gundlach, Plast Reconstr Surg 2005).
- Young patients might inadvertently manipulate the conjunctival expander, resulting in it being dislodged from the fornix.
- Prevention and Management
- Meticulous technique
- Careful implant measurements
- Inflatable saline expanders
- Intraoperative
- Same as nonexpandable implants
- Postoperative
- Expander or injector leakage
- Placement of the expander beneath the periosteum instead of in the intraconal space might result in thinning of the orbital roof and deformation of the orbital cavity (Dunaway, Br J Plast Surg 1996).
- Prevention and Management
- If leak is minimal, can keep expander in place and continue injections/expander exchange
- Expander exchange
Historical perspective
Enucleation is one of the oldest surgeries in ophthalmology, being first reported by George Bartish in the 16th century (Luce, Int Ophthalmol Clin 1970). The procedure at that time was done without anesthesia. The globe was sharply dissected out of the orbit, leaving a socket that was deformed and often infected.
Modern enucleation techniques date back to the 1840s followed by the use of glass orbital implants called “Mules” spheres (Mules, Trans Ophthalmol Soc UK 1885). Complications with glass spheres such as extrusion and shattering led to the development of other implant materials.
About 10,000 enucleations are performed in the United States each year. The most common indications are blind painful eye, tumor, and trauma (Erie, Am J Ophthalmol 1992).
References and additional resources
- AAO, Basic and Clinical Science Course. Section 7: Orbit, Eyelids, and Lacrimal System, 2013-2014.
- AAO, Ophthalmology Monographs 8. Surgery of the Eyelid, Orbit & Lacrimal system, Vol. 3, 1995.
- AAO, Focal Points: The Anophthalmic Socket, Module #9, 1992.
- AAO, Focal Points: Choroidal Melanoma Update, Module #4, 2005.
- Choi YJ, Park C, Jin HC, Choung H-K, Lee MJ, K N, Khwarg SI, Yu YS. Outcome of smooth surface tunnel porous polyethylene orbital implants (Medpor SST) in children with retinoblastoma. Br J Ophthalmol. 2013; 97:1530-1533.
- Christel PS. Biocompatibility of surgical-grade dense polycrystalline alumina. Clin Orthop Relat Res. 1992; 282:10-18.
- Custer PL, Kennedy RH, Woog JJ, Kaltreider SA, Meyer DR. Orbital implants in enucleation surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2003; 110:2054-2061.
- Dunaway DJ, David DJ. Intraorbital tissue expansion in the management of congenital anophthalmos. Br J Plast Surg. 1996; 49:529
- Erie JC, Nevitt MP, Hodge D, Ballard DJ. Incidence of enucleation in a defined population. Am J Ophthalmol. 1992; 113:138-144.
- Esmaeli B, Elner SG, Schork MA, Elner VM. Visual outcome and ocular survival after penetrating trauma: a clinicopathologic study. Ophthalmology. 1995; 102:393-400.
- Fay A, Nallasamy N, Pemberton JD, Callahan A, Wladis EJ, Nguyen J, Durand ML; New England Oculoplastics Society Study Group. Prophylactic postoperative antibiotics for enucleation and evisceration. Ophthal Plast Reconstr Surg. 2013; 29:281-285.
- Gundlach KKH, Guthoff RF, Hingst VHM, Schittkowski MP, Bier UC. Expansion of the socket and orbit for congenital clinical anophthalmia. Plast Reconstr Surg. 2005; 116:1214-1222.
- Heher KL, Katowitz JA, Low JE. Unilateral dermis-fat graft implantation in the pediatric orbit. Ophthal Plast Reconstr Surg. 1998; 14(2):81-88.
- Hou Z, Yang Q, Chen T, Hao L, Li Y, Li D. The use of self-inflating hydrogel expanders in pediatric patients with congenital microphthalmia in China. J AAPOS. 2012; 16:458-463.
- Huang D, Yu Y, Lu R, Yang H, Cai J. A modified evisceration technique with scleral quadrisection and porous polyethylene implantation. Am J Ophthalmol. 2009; 147:924-928.
- Iordanidou V, De Potter P. Porous polyethylene orbital implant in the pediatric population. Am J Ophthalmol. 2004; 138:425-429.
- Jordan DR, Gilberg S, Mawn LA. The bioceramic orbital implant: experience with 107 implants. Ophthal Plast Reconstr Surg. 2003; 19:128-135.
- Jordan DR, Klapper SR. Surgical techniques in enucleation. The role of various types of implants and the efficacy of pegged and nonpegged approaches. Int Ophthalmol Clin. 2006; 46(1):109-132.
- Leaf N, Zarem H. Correction of contour defects of the face with dermal and dermal-fat grafts. Arch Surg. 1972; 105:715-719.
- Lee V, Suback-Sharpe I, Hungerford JL, Davies NP, Logani S. Exposure of primary orbital implants in post enucleation retinoblastoma patients. Ophthalmology. 2000; 107:940-946.
- Luce CM. A short history of enucleation. Int Ophthalmol Clin. 1970; 10:681-687.
- Malhotra R. Deep orbital Sub-Q restylane (nonanimal stabilized hyaluronic acid) for orbital volume enhancement in sighted and anophthalmic orbits. Arch Ophthalmol. 2007; 125:1623-1629.
- Mawn LA, Jordan DR, Gilberg S. Proliferation of human fibroblasts in vitro after exposure to orbital implants. Can J Ophthalmol. 2001; 36:245-251.
- Mitchell KT, Hollsten DA, White WL, O’Hara MA. The autogenous dermis-fat orbital implant in children. J AAPOS. 2001; 5:367-369.
- Moshfeghi DM, Moshfeghi AA, Finger PT. Enucleation. Surv Ophthalmol. 2000;44:277-301.
- Mules PH. Evisceration of the globe, with artificial vitreous. Trans Ophthalmol Soc UK. 1885; 5:200-206.
- Nerad JA. Enucleation, Evisceration, and Exenteration: The Care of the Eye Socket. In: Oculoplastic Surgery, The Requisites. St. Louis: Mosby, 2001: 419-443.
- Patel BC, Sapp NA, Collin R. Standardized range of conformers and symblepharon rings. Ophthal Plast Reconstr Surg. 1998;14:144-145.
- Riebel O. Plastic surgery on the upper eye-lid after enucleation of the eye. Br J Ophthalmol. 1976; 60:726-727.
- Rosen HM. The response of porous hydroxyapatite to contiguous tissue infection. Plast Reconstr Surg. 1991; 88:1076-1080.
- Rubin PAD. Enucleation, evisceration, and exenteration. Curr Opin Ophthalmol. 1993; 4:39-48.
- Shah SU, Shields CL, Lally SE, Shields JA. Hydroxyapatite orbital implant in children following enucleation: analysis of 531 sockets. Ophthal Plast Reconstr Surg. 2015; 31:108-114.
- Smith B, Bosniak S, Lisman R. An autogenous kinetic dermis-fat orbital implant. Ophthalmology. 1982; 89:1067-1071.
- Smith B, Lisman RD. Use of sclera and liquid collagen in the camouflage of superior sulcus deformities. Ophthalmology. 1983; 90:230-235.
- Spivey BE, Allen L, Stewart WB. Surgical correction of superior sulcus deformity occurring after enucleation. Am J Ophthalmol. 1976; 82:365-370.
- Su GW, Yen MT. Current trends in managing the anophthalmic socket after primary enucleation and evisceration. Ophthal Plast Reconstr Surg. 2004; 20:274-280.
- Zamani M, Thyagarajan S, Olver JM. Adjunctive use of hyaluronic acid gel (Restylane Sub-Q) in anophthalmic volume deficient sockets and phthisical eyes. Ophthal Plast Reconstr Surg. 2010; 26:250-3.
