Soft-Tissue Fillers
Updated July 2025
Establishing the diagnosis
Etiology
Aging is characterized by volume deflation, facial descent and surface irregularities in the skin.
Dermal fillers treat volume loss, improve facial support, and reduce skin irregularities.
Scars may also be treated with soft-tissue fillers.
Use of an injectable agent for cosmetic enhancement was reported in 1899 by Robert Gersuny, who injected paraffin to create a testicular prosthesis (Kontis, Facial Plast Surg 2009). Materials with better biocompatibility are now available.
Epidemiology
- Age-related volume loss and skin surface irregularities
- Severity of aging relates to intrinsic factors (genetics) and extrinsic factors of ultraviolet exposure, smoking, diet and lifestyle choices.
- Society favors a youthful appearance. Improved facial appearance contributes to psychological well-being and to positive social function.
History
- Have patient identify areas of concern.
- History of previous surgery or filler treatment
- Current medications and supplements, especially blood thinners and anti-platelet agents
- Medical history and allergies including prior response and reactions to similar products, underlying autoimmune disease, HSV infection and pregnancy
- Informed consent should be obtained.
Testing
- Photographs to document before and after treatment
- Skin testing for allergy not generally indicated except for historic materials such as bovine collagen
Patient management: treatment and follow-up
- Clear discussion regarding risks and benefits of fillers for nonsurgical rejuvenation. Patient should understand the limitations of fillers, and that it does not replace surgery or laser resurfacing.
- Topical anesthetic and nerve blocks are optional as most fillers are premixed with anesthetic. Ice packs are helpful for patient comfort, both before and after treatment.
- Application of filler
- Fillers are placed in various levels of the cutaneous structures depending on the depth of the line or fold and the product properties.
- Thinner gels are placed in the superficial dermis to diminish superficial rhytides.
- Thicker gels are placed in the deep dermis to soften and eliminate folds (Figures 1 and 2).
- Thicker materials and hydroxyapatite michrospheres are placed in the preperiosteal plane to modify shape and volume (Figures 3 and 4).
- Permanent products should be placed in deeper layers.
- Fillers not only soften rhytides, but can also stimulate collagen production (Carruthers, Dermatol Surg 2014).
- Poly L-lactic acid is injected at the dermal-subcutaneous junction and deeper; it works by stimulating an inflammatory reaction that induces fibroblast activity to create new collagen and cellular remodeling.
- Calcium hydroxylapatite microspheres induce a histiocytic and fibroblastic response, resulting in collagen formation.
- Hyaluronic acid gel has also been shown to induce collagen formation (Wang, JAMA Derm 2007).
- Patient should always be seated upright rather than supine to best demonstrate the areas of rhytids.
- Be sure the head is supported comfortably to avoid patient movement during injection.
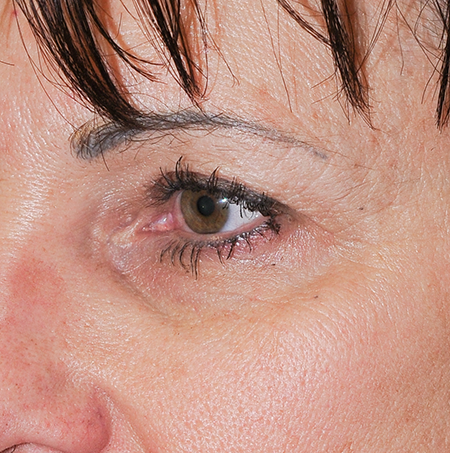
Figure 1. Before Restylane to tear trough and Perlane to medial cheek.
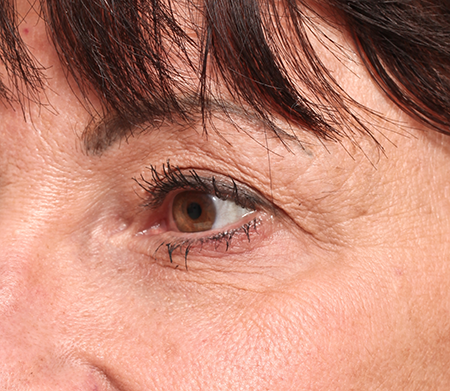
Figure 2. After Restylane to tear trough and Perlane to medial cheek.

Figure 3. Before Juvederm Voluma to outer cheek.

Figure 4. After Juvederm Voluma to outer cheek.
- Clinical products
- Hyaluronic acid (HA): naturally occurring polysaccharide that is biodegradable, biocompatible and nonimmunogenic; low potential for allergies; consistent across species; draws water and consequently adds volume (Carruthers, Dermatol Surg 2014, see also articles by Kontis, Anderegg, Bentkover)
- Restylane, Restylane-L, and Restalyn Lyft (Galderma)
- Derived from bacterial fermentation without animal source
- Restylane Silk, Restylane, and Restylane Lyft all have the same hyaluronic acid concentration of 20 mg/ml and are biphasic — particles of crosslinked HA suspended in a liquid. However, they comprise different particle sizes, with Restylane Silk, Restylane, and Restylane Lyft containing 200,000, 100,000, and 10,000 particles per milliliter, respectively.
- Restylane Silk can be injected more superficially than Restylane Lyft in the dermis or dermal-subcutaneous tissue junction. It can also be injected through deeper planes for nasolabial folds, rhytides, scars, and lip augmentation.
- Similar higher molecular weight product Restylane Lyft is used for deeper volume augmentation.
- Typical correction lasts 6–12 months, depending on the area treated.
|
Restylane Fine Lines |
Biphasic HA |
Elasticity 12,13 |
Particle size |
20mg/mL |
|---|---|---|---|---|
|
Restylane |
Biphasic HA |
513 |
250 microns |
20 mg/cc |
|
Restylane Lyft |
Biphasic HA |
541 |
500 microns |
20mg/mL |
- Juvederm Ultra, Juvederm Ultra Plus, Voluma, and Volbella (Bentkover, Facial Plast Surg 2009; see also articles by Dayan, Hassouneh, Carruthers, Kontis, Palm, Sundaram, Montes)
- Derived from bacterial fermentation without animal source
- Ultra formulation contains high hyaluronic acid concentration (24 mg/ml) and cross-linked with persistence up to 1 year.
- Monophasic: stable pure gel without particles
- Voluma can last up to 2 years.
|
Juvederm Ultra |
Monophasic |
Elasticity 28 |
24mg/cc |
|
Juvederm Ultra Plus |
Monophasic |
75 |
24 mg/mL |
|
Voluma |
Monophasic |
274 |
20 mg/mL |
|
Volbello |
Monophasic |
|
15 mg/mL |
- RHA Collection (Revance Aesthetics)
- Derived from bacterial fermentation
- Indicated for moderate-to-severe perioral, nasolabial folds and marionette rhytids1
- It has a hyaluronic acid filler with lidocaine and is cross-linked with persistence of up 12-15 months dependent upon the product selected
- RHA Redensity
- hyaluronic acid concentration of 15 mg/g that persists up to 12 months
- Indicated for injection into dermis and superficial dermis of the face for perioral rhytids
- RHA 2
- hyaluronic acid concentration of 23 mg/g
- Indicated for injection into mid-to-deep dermis for dynamic rhytids such as the nasolabial folds
- RHA 3
- hyaluronic acid concentration of 23 mg/g
- While the concentration of hayluranic acid is the same as RHA 2 this has a higher viscosity making it more conducive to the correction of deep rhytids
- Indicated for injection into mid-to-deep dermis for dynamic rhytids (ex. Marionette lines)
- RHA 4
- hyaluronic acid concentration of 23 mg/g
- Most heavily cross-linked and more viscous than RHA 2 or RHA 3
- I indicated for injection in the deep dermis to superficial subcutaneous tissue to build volume
- Revanesse (Prollenium Medical Technologies)
- Derived from bacterial fermentation with streptococcal cultures
- It has a hyaluronic acid concentration of 22-28 mg/ml with 3 mg/ml of lidocaine and is cross-linked with persistence of up to 1 year
- Indicated for moderate-to-severe facial rhytids and is to ge injected into the mid to deep dermis
- Boltero Balance (Merz)
- Derived from bacterial fermentation with streptococcal cultures
- It has an hyaluronic acid concentration of 22.5 mg/ml and is cross-linked with persistence of 6 months
- Indicated for correction of moderate-to-severe facial wrinkles and is to be injected into the mid to deep dermis
- Monophasic: cohesive gel with variable density
- Nonhyaluronic acid fillers
- Radiesse (Merz) (Kontis, Facial Plast Surg 2013; see also Bentkover, Hassouneh)
- Calcium hydroxylapatite microspheres, 25–45 microns in diameter suspended in carboxymethylcellulose carrier
- Induces neocollagenesis and provides scaffold for tissue with longer-lasting (2–5 years) effect
- Initially approved for vocal cord augmentation and repair of oromaxillofacial defects, then subsequently approved as dermal filler for moderate to severe nasolabial folds in 2006
- Deep treatment to nasolabial folds and other deep sites; white product with tendency to clumping discourages treatment in the lips or tear trough.
- Sculptra (Galderma) (Woerle, J Drugs Dermatol 2004; see also Bentkover, Kontis)
- Poly-L-lactic acid reconstituted with sterile water to create a hydrogel with a methylcellulose carrier
- Promotes gradual collagen production and cellular remodeling around dispersed product with a foreign body reaction and dermal fibrosis
- Initially approved by FDA for HIV lipoatrophy in 2004; approved for cosmetic use and facial volume augmentation in 2009
- 2–3 treatments 4–6 weeks apart to achieve desired endpoint with facial volumination
- Dilution and dispersion necessary to prevent granuloma formation
- Artefill (Suneva Medical) (Kontis, Facial Plast Surg 2013; see also Hassouneh, Bentkover)
- Bovine collagen with suspended polymethyl methacrylate (PMMA) microspheres
- Potential for long term volume augmentation
- Allergy testing (at least 1 month before) for bovine collagen required before treatment
- FDA-approved in 2006 and initially distributed Artes Medical
- Liquid silicone (Silikon-1000, Alcon) (Bentkover, Facial Plast Surg 2009)
- Above approved in vitreoretinal surgery
- Long history of use of similar silicone products as superficial dermal fillers; not FDA approved
- History of abuse and chronic lipogranuloma formation along with other safety questions contraindicates use.
Preventing and managing treatment complications
(Hassouneh, Facial Plast Surg Clin N Am 2013; see also Fischer, Bentkover, Montes, Woerle, Gladstone, Niamtu, Christensen, Sharad)
Common and less serious
- Bleeding and bruising
- Prevented by discontinuation of aspirin and other nonsteroidal anti-inflammatory drugs, vitamin E, herbals such as gingko biloba, St. John’s wort, ginseng, ginger, and garlic
- Use of ice and arnica can help minimize bruise.
- Use of fine needles or blunt cannulas can help avoid unnecessary bruising.
- Allergic reactions
- Rare in most fillers
- Allergic reactions may include delayed hypersensitivity reaction with redness and swelling to rare anaphylaxis.
- Chronic inflammation responds to systemic or intralesional corticosteroids and lessens with time.
- Bacterial infection
- Infection is a rare complication and the most likely pathogens are skin flora.
- Heralded by increasing pain and erythema at injection site beginning 24–72 hours after treatment
- Cultures and appropriate treatment indicated
- Biofilms have been implicated and can be prevented by thorough cleansing of the area with antiseptic solution such as stanhexidine and local application of topical antibacterial agent.
- Viral infection
- Usually reactivation of herpes simplex virus (occasionally varicella zoster virus) in the perioral area
- Potential to prevent with antiviral prophylaxis Valcyclovir 500 mg BID, begin 2 days pretreatment, continue 5 days post-treatment
- Visible filler with beading or pooling of product
- Avoided with appropriate placement and quantity of injection
- Generally resolves with time, but may needle overlying skin and express product if a persistent problem
- Over-augmentation
- May respond to observation or needling and expressing
- Hyaluronate fillers (Restylane, Juvederm) can be treated with small injections of hyaluronidase into the overfilled area and will be broken down and removed in 3–48 hours.
- Report of hemifacial spasm secondary to facial nerve compression after temple filler (Kishi, Ophthal Plast Reconstr Surg 2025)
Serious
- Tissue necrosis: possibly from vascular compression in area of injection or if product is placed too superficially into the epidermis; white blanching of skin and resistance to injection may indicate wrong level of injection.
- Filler should be dissolved immediately with hyaluronidase (Kroumpouzos, JMIR Dermatol 2024)
- Embolic event: rare and due to direct intravascular injection with resulting tissue necrosis, CRAO, or other ischemic event in the head and neck area where the product was injected
- Vision loss is rare but has been reported after as little as 0.2mL filler volume. Vision loss is typically unilateral and occurs immediately after injection. The highest risk areas are the nose, glabella and forehead (Kapoor, Aesthetic Plast Surg 2020)
- Avoid by withdrawing the plunger slightly before injecting (aspiration technique), particularly in areas of known large vessels in the glabella and medial canthus.
- As above, complications should be managed with immediate injection of hyaluronidase. However, the success of retrobulbar hyaluronidase is limited (Fakih-Gomez, Aesthetic Plast Surg 2025)
- Some doctors recommend keeping nitroglycerin paste on hand; however there is no clinical evidence that vasodilation helps (Hwang, Ophthal Plast Reconstr Surg. 2016 )
- Severe complications are rare overall: one large study found a serious complication rate of 0.0041%. The majority of embolic events were from high-risk areas such as the forehead (Tamura, Ann Plast Surg 2025)
Patient instructions
- Avoidance of blood thinners and nonsteroidal anti-inflammatory drugs pretreatment
- Ice and elevation of treated area immediately post-treatment for 24–48 hours
- Sculptra treatment requires massage of injected area for several days.
- Soft lumps or bulges after hyaluronate or other product treatment can be minimized with gentle massaging of the area to disperse the product.
References and additional resources
- Anderegg U, Simon JC, Averbeck M. More than just a filler – the role of hyaluronan for skin homeostasis. Exp Dermatol 2014; 23:295.
- Bentkover SH. The biology of facial fillers. Facial Plast Surg 2009; 25: 73.
- Buck DW, Alam M, Kim JYS. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg 2009; 62:11.
- Carruthers JDA, Carruthers JA, Humphrey S. Fillers and Neocollagenesis. Dermatol Surg 2014; 40:S134.
- Christensen L, et al. Adverse reactions to injectable soft tissue permanent fillers. Aesth Plast Surg 2005; 29:34.
- Dayan S, Arkins JP, Somenek M. Restylane persisting in lower eyelids for 5 years. J Cosmet Dermatol 2012;11:237.
- Fakih-Gomez N, Muñoz-Gonzalez C, Porcar Plana CA, Puzo Bayod M, Madero J. Retrobulbar hyaluronidase in hyaluronic acid-induced ocular vascular occlusion: efficacy, challenges, and implications for clinical practice. Aesthetic Plast Surg. 2025;49(5):1458-1468.
- FAQs. Belotero BALANCE. Accessed April 5, 2023. https://www.belotero.com/faqs/
- Fischer TC. A European evaluation of cosmetic treatment of facial volume loss with JuvedermTM VolumaTM in patients previously treated with Restylane Sub-QTM. J Cosmet Dermatol 2010; 9:291.
- Gladstone HB, Cohen JL. Adverse effects when injecting facial fillers. Semin Cutan Med Surg 2007; 26:34.
- Hassouneh B, Newman JP. Lasers, Fillers, and Neurotoxins. Avoiding complication in the cosmetic facial practice. Facial Plast Surg Clin N Am 2013;21:585.
- Health C for D and R. Revanesse® Lips+ – P160042/S010. FDA. Published online February 4, 2021. Accessed April 5, 2023. https://www.fda.gov/medical-devices/recently-approved-devices/revanesser-lips-p160042s010
- Health C for D and R. RHA Redensity – P170002/S012. CDRH. Published online January 19, 2022. Accessed April 4, 2023. https://www.fda.gov/medical-devices/recently-approved-devices/rha-redensity-p170002s012
- Hwang CJ, Morgan PV, Pimentel A, Sayre JW, Goldberg RA, Duckwiler G. Rethinking the role of nitroglycerin ointment in ischemic vascular filler complications: an animal model with icg imaging. Ophthalmic Plast Reconstr Surg. 2016;32(2):118-122.
- Kapoor KM, Kapoor P, Heydenrych I, Bertossi D. Vision loss associated with hyaluronic acid fillers: a systematic review of literature. Aesthetic Plast Surg. 2020;44(3):929-944.
- Kishi A, Gregerson CH, Yang C, Varma H, Akella SS. Bilateral hemifacial spasm after filler injection. Ophthalmic Plast Reconstr Surg. Published online June 9, 2025.
- Kontis TC. Contemporary Review of Injectable facial fillers. Facial Plast Surg 2013; 15:58.
- Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg 2009; 25:67.
- Kroumpouzos G, Treacy P. Hyaluronidase for dermal filler complications: review of applications and dosage recommendations. JMIR Dermatol. 2024;7:e50403.
- Montes JR. Volumetric considerations for lower eyelid and midface rejuvenation. Curr Opin Ophthalmol 2012; 23:443.
- Muhn C et al. The evolving role of hyaluronic acid fillers for facial volume restoration and contouring: a Canadian overview. Clin Cosmet Invest Dermatol 2012; 5:147.
- Niamtu J. Complications in Fillers and Botox. Oral Maxillofac Surg Clin North Am 2009; 21:13.
- P090016c.pdf. Accessed April 5, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf9/P090016c.pdf
- P170002C.pdf. Accessed April 5, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170002C.pdf
- Palm MD. Filler frontier: what’s new and heading west to the US market. Semin Cutan Med Surg 2014; 33:157.
- The RHA® Collection | Dynamic Facial Fillers. Accessed April 4, 2023. https://rhacollection.com/
- Sandashivaiah AB, Mysore V. Biofilms: their role in dermal fillers. J Cutan Aesthet Surg 2010; 3:20.
- Sharad J. Dermal fillers for the treatment of tear trough deformity: A review of anatomy, treatment techniques, and their outcomes. J Cutan Aesthet Surg 2012; 5:229.
- Sundaram H, Voights B, Beer K, Meland M. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxyl apatite and hyaluronic acid. Dermatol Surg 2010; 35:1859.
- Tamura T, Tamura T, Okumura K, Funakoshi Y, Teranishi H. Serious complications of hyaluronic acid fillers-a retrospective study of 290,307 cases. Ann Plast Surg. 2025;94(6):630-633.
- Teosyal RHA. Toronto Facial Plastic Surgery and Laser Centre | Dr. Torgerson. Accessed April 4, 2023. https://drtorgerson.com/non-surgical-procedures/dermal-fillers-toronto/teosyal-rha/
- Wang F et al. In Vivo Stimulation of De Novo Collagen Production Caused by Cross-linked Hyaluronic Acid Dermal Filler Injections in Photodamaged Human Skin. Arch Dermatol. 2007;143(2):155-163.
- Woerle B, Hanke CW, Sattler G. Poly-L-Lactic Acid: A temporary filler for soft tissue augmentation. J Drugs Dermatol 2004; 3:385.
Financial disclosures
Financial Disclosures
Reviewers
Victoria North – No disclosures
