Horner Syndrome
Updated May 2024
Establishing the diagnosis
Etiology
- Disruption of the sympathetic pathway that supplies the head, eye, and neck (Figure 1)
- First-order neuron (FON): Fibers originate from the posterolateral hypothalamus traveling to the ciliospinal center of Budge-Waller located in the C8-T2 region of the cervical and thoracic spine.
- Second-order neuron (SON): Fibers originate from the cervical spine and travel through the brachial plexus, over lung apex, up to superior cervical ganglion, which is intimately situated with the internal carotid artery (ICA).
- Third-order neuron (TON): Fibers originate from the superior cervical ganglion and travel along the internal carotid artery, through cavernous sinus, adjacent to CN 6, to the Muller’s muscle (upper eyelid), the inferior tarsal muscle (lower eyelid), and pupillary dilator muscles.
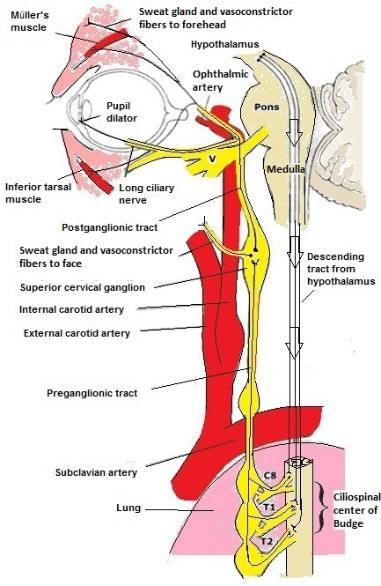
Figure 1. Sympathetic pathway for pupillary innervation. Modified from Martin, TJ, Corbett, JJ. The Pupil. In: Neuro-ophthalmology: the requisites in ophthalmology, Krachmer, JH (Ed), St. Louis, MO: Mosby; 2000.
Epidemiology in adults
- First-order lesions
- Lateral medullary infarction (Wallenburg syndrome)
- Cerebellar ataxia
- Loss of pain and temperature over one side of face
- Vertigo
- Dysarthria, dysphagia
- Hypothalamus insult (central Horner syndome)
- Infarction
- Hemorrhage
- Tumor
- Vertebrobasilar insufficiency
- Vertebral artery dissection
- Tumors
- Demyelinating lesions
- Syringomyelia
- Cervical cord trauma
- Cervical disk disease and osteoarthritis
- Second-order lesions
- Trauma/surgery of spinal cord or brachial plexus
- Apical lung tumors (Pancoast tumor)
- Lumbar epidural anesthesia (most often with obstetrical procedures)
- Chiropractic manipulation
- Third-order lesions
- Internal carotid dissection
- Traumatic or spontaneous
- Due to a disruption of layers of the artery causing blood to collect between the layers (Lyrer, Neurology 2014)
- Horner syndrome (HS) is seen in nearly 40% of internal carotid artery dissection (ICAD) (Lyrer, Neurology 2014).
- Average age of ICAD with HS 40–50 years old
- 65% males
- In one study of patients with ICAD, the risk of stroke or TIA was less in those with HS compared to those without.
- Other ophthalmologic manifestations (Biousse, Am J Ophthalmol 1998)
- 52% of patients with ICAD presented with ophthalmologic symptoms or signs.
- Monocular visual loss 28%
- 76% of those were painful.
- Scintillations
- Ischemic optic neuropathy
- Diplopia
- Other ICA lesions: thrombosis, endarterectomy, stenting, radical neck surgery, lymphadenopathy, penetrating trauma
- Cavernous sinus lesion
- Tumor extension, including nasopharyngeal carcinomas (Murchison, Ophthal Plast Reconstr Surg 2009)
- Pituitary lesions
- Herpes zoster
- Tolosa-Hunt syndrome
- Otitis media
- Cluster headaches (HS lasts less than 1–2 hours)
- Giant cell arteritis (Shah, Eye (Lond) 2007)
- Traditional tonsil excision or radiofrequency tonsil ablation (Kucur, Case Rep Otolaryngol, 2015)
- Eagle syndrome (pressure from an elongated styloid process on the carotid artery) (Chang, J Neuroophthalmol, 2015)
- Idiopathic
Epidemiology in children
- Congenital and acquired
- Acquired
- Neuroblastoma
- Presenting sign of 2% of patients with neuroblastoma (Musarella, Ophthalmology 1984)
- In children presenting with HS, 33% had a tumor and 22% had a neuroblastoma (Mahoney, Amer J Ophthal 2006).
- Head, neck, and chest surgery
- Infection
- Congenital
- Birth trauma
- Idiopathic: Approximately 67% of patients presenting without a known cause of the HS were found to be idiopathic (Mahoney, Amer J Ophthal 2006).
- Vascular anomaly
- Neoplasm
- The Mahoney et al. study included 2 patients born with HS that was caused by masses, 1 being a neuroblastoma and 1 neurofibroma.
- They also reported a patient with a history of birth trauma who was worked up with an MRI and also found to have a neuroblastoma.
History
- Presence of pain (neck pain, facial pain, headache)
- Pain is very suggestive for a carotid artery dissection and is reported to occur up to 75% of the time (Davagnanam, Eye 2013).
- History of trauma
- Important to consider that HS can be masked by concurrent traumatic mydriasis and traumatic ptosis (Wessel, Ophthal Plast Reconstr Surg 2011)
- Review of old patient photographs
- Very frequently a “new” anisocoria can be seen in childhood photos.
- Brainstem signs
- Diplopia, vertigo, ataxia, lateralized weakness or sensory loss
- Suggestive of a first-order lesion
- Bilateral muscle or sensory weakness, bowel/bladder impairment
- Suggestive cervicothoracic cord injury (first-order lesion)
- Arm pain/weakness
- Brachial plexus injury (second-order lesion)
- Particularly important in the assessment of pediatric patients
- Discuss birth history, complications, forceps use
- Chiropractic manipulation
- Several reports of subsequent carotid dissection leading to HS (Parwar, Am J Ophthalmol 2001)
- An especially important consideration for patients younger than 45 years old (given the increased risk of ICAD)
- Diplopia
- Particularly ask if worse in lateral gaze, suggestive of CN VI palsy
- CN VI palsy due to cavernous sinus lesion (third-order lesion)
- CN VI is most commonly affected, presumably due to its proximity to the ICA and given that it is suspended within the cavernous sinus.
- Other cranial nerves located within the cavernous sinus can also be affected (CN III, IV, V1, V2).
Clinical features
(Davaganam, Eye 2013)
- Classic triad of ptosis, miosis and facial anhidrosis (Figure 2)
- Mild upper-lid ptosis (1–3 mm)
- Ptosis can be subtle and reportedly absent in 12% of HS patients (Maloney, Am J Ophthalmol 1980).
- Lower-eyelid elevation (“reverse ptosis”)
- Due to denervation of the inferior tarsal muscle (lower-eyelid analogue of Muller’s muscle)
- Appearance of enophthalmos due to narrowed vertical palpebral aperture
- Pupillary miosis
- Anisocoria is greater in the dark.
- Dilation lag: slower redilation on removal of light
- Ipsilateral facial anhidrosis
- Only present in first- and second-order lesions, as facial sweat-gland fibers branch off at the superior cervical ganglion
- Variable presentation: Involvement ranges from entire half of face to small patch on the forehead, depending on lesion location.
- Evaluation
- Sweat friction test (Rosenberg, Am J Ophthalmol 1989)
- A standard prism bar and the forehead are cleaned with an alcohol pad and allowed to dry.
- The bar is then placed flat against 1 side of the forehead and drawn down with mild pressure.
- The same is done on the other side.
- The side with anhidrosis will provide significantly less resistance compared to the normal side.
- Starch-iodine test
- Iodine is applied to an area and allowed to dry.
- A thin layer of starch is then applied.
- Areas of anhidrosis will appear a dark bluish color.
- This can present as impaired facial flushing (Harlequin sign) in children.
- Iris heterochromia
- Secondary to a congenital Horner syndrome, where ipsilateral iris is lighter
- Occurs in approximately 12% of pediatric HS (Mahoney, Arch Ophthalmol 2011)
- Other signs: ipsilateral conjunctival injection, nasal stuffiness, increased near point of accommodation
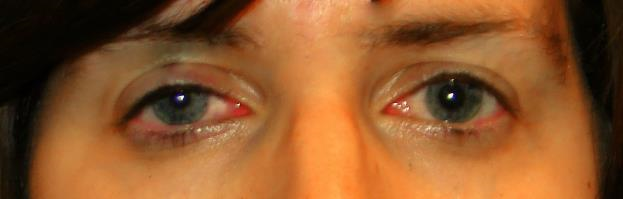
Figure 2. Patient with right HS. Note the mild ptosis of the right-upper lid and the miosis of the right pupil.
Testing for miosis due to HS
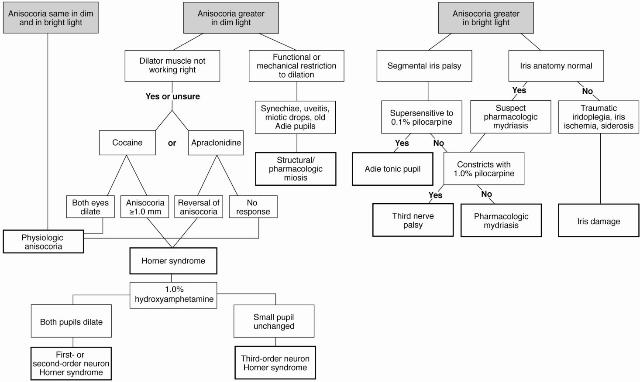
Figure 3. Anisocoria flowchart http://one.aao.org/image/anisocoria-evaluation.
Cocaine (2%–10%) dilates unaffected eye, increasing anisocoria.
- Cocaine blocks reuptake of catecholamines (norepinephrine).
- Norepinephrine is not released in impaired sympathetic innervation, therefore cocaine has no effect on HS eyes.
- Reported techniques for number and timing of drops is highly variable.
- Kardon et al., in their classic study in 1990, placed 1 drop bilaterally and evaluated the pupils 50–60 minutes later.
- The current AAO recommendation in the neuro-ophthalmology volume of the BCSC states 2 drops of cocaine 4% or 10% should be placed and evaluate for anisocoria in 45 minutes.
- Typically the pupils are tested in dim lighting, however, a comparison study has not been done.
- Most studies use cocaine 10%, but other concentrations have been documented.
- 4% cocaine has been shown to be effective in a pediatric study (Chen, J Ocul Pharmacol Ther 2006).
- Lower concentrations will likely be as efficacious, however, it might take longer to see the effect.
- Anisocoria postcocaine of 0.8 or greater is diagnostic (Kardon, Arch Ophthalmol 1990),
- Drawbacks of cocaine testing (Smit, S Afr Med J 2010)
- Becoming increasingly difficult to acquire
- Expensive
- Often requires pharmacy to compound
- 4% cocaine is reportedly available commercially as a preservative-free drop.
- Schedule status
- Leads to pharmacies resistant to carrying the drug and requires that it be kept secure in a locked container and a record kept of drops administered
- Due to inhibition of reuptake of hydroxyamphetamine, localization testing using hydroxyamphetamine cannot be done on the same day.
- Most studies recommend waiting at least 24 hours.
- Urine drug screen positive after ophthalmic cocaine (Jacobson, Am J Ophthalmol 2001)
- 94% positive at 4 to 6 hours
- 70% positive at 24 hours
- 2% positive at 48 hours
- Decreased sensitivity if there is partial interruption of sympathetic chain
- Response in patients with dark irides can take up to 3 hours.
Apraclonidine (0.5% or 1%) reverses anisocoria.
- Practical alternative to cocaine with a comparable sensitivity of 87% (Davagnanam, Eye 2013)
- Less expensive and widely available
- Benefit of having its effect on the abnormal pupil
- Primarily used for management of postsurgical/laser intraocular pressure elevation through its alpha-2 agonist activity
- Alpha-1 agonist activity weakly dilates a normal pupil.
- Due to denervation hypersensitivity in HS, the upregulated alpha-1 receptors result in pupil dilation.
- Reported to take at least 36 hours for supersensitivity to apraclonidine to develop (Lebas, J Neuroophthalmol 2010).
- Therefore apraclonidine testing should not be done on an acute case of HS and might cause deleterious delays in further workup and detection of the etiology.
- Technique
- 1 drop in each eye; typically evaluate in 1 hour.
- If using in a child, consider punctal occlusion.
- Anisocoria following apraclonidine is most appreciable with ambient light (Chen, Am J Ophthalmol 2006).
- Drawbacks of apraclonidine testing (Smit, S Afr Med J 2010)
- Must wait at least 36 hours after development of HS
- Side effects in pediatric population
- Not recommended for use in patients less than 6 months old
Localization of lesion
1% hydroxyamphetamine (Paradrine) dilates normal pupils by releasing norepinephrine from the postganglionic nerve endings.
- Dilation if third-order neuron is intact
- No dilation if third-order neuron is involved
- Drawbacks of hydroxyamphetamine
- Cannot be used on same day as cocaine/apraclonidine testing; must wait 24–48 hours
- Not reliable in children
- Becoming increasingly more difficult to acquire
- High false-negative rate, particularly in the acute phase, because it takes time for the TON to become depleted on norepinephrine (Van der Wiel, J Neurol Sci 1983)
Due to the drawbacks of hydroxyamphetamine, phenylephrine 1% is used increasingly.
- Easily prepared by diluting the readily available 2.5% or 10% phenylephrine solutions
- Also based on supersensitivity of alpha receptors from denervation
- Causes dilation of TON, although causing no dilation of a FON and only minimal dilation of a SON or normal pupil
- A study of 14 patients, 11 with postganglionic HS, showed phenylephrine 1% had a sensitivity of 81% and specificity of 100% compared to hydroxyamphetamine sensitivity of 93% and specificity of 83% (Danesh-Meyer, Br J Ophthalmol 2009).
- Drawbacks of phenylephrine 1%
- Does not dilate the normal pupil, so one cannot determine whether lesion is preganglionic or drops are ineffective
- Relies on denervation supersensitivity, which takes time and is variable if there is partial denervation
Testing for miosis due to HS in children
- Cocaine versus apraclonidine
- Large studies have established cocaine as a useful tool in the diagnosis of HS (Kardon, Arch Ophthalmol 1990), however the use of apraclonidine is recent and studies are limited (Chen, Am J Ophthalmol 2006).
- Significant concerns surround the use of apraclonidine in children due to the known side-effect profile of the similar drop brimonidine and documented cases of side effects of apraclonidine with HS testing
- There have been reports of side effects following HS testing with apraclonidine including (Watts, J AAPOS. 2007)
- Somnolence
- Bradycardia
- Hypotension
- Apnea
- As a result of the documented concerns of apraclonidine testing in children, most recommend the use of cocaine if possible. If cocaine is unavailable, Watts et al. recommend monitoring the child for at least 2 hours after testing with apraclonidine.
- Hydroxyamphetamine
- Often not done due to drawbacks
- High false-positive in congenital cases (Weinstein, Arch Ophthalmol 1980)
- Believed to occur in neonates due to damage to preganglionic neurons causing damage to postganglionic neurons
- High false-negative and lack of specificity in children
- Localization is arguably less important in the pediatric population because further work-up is typically the same, regardless of the neuron order affected.
Testing to establish diagnosis
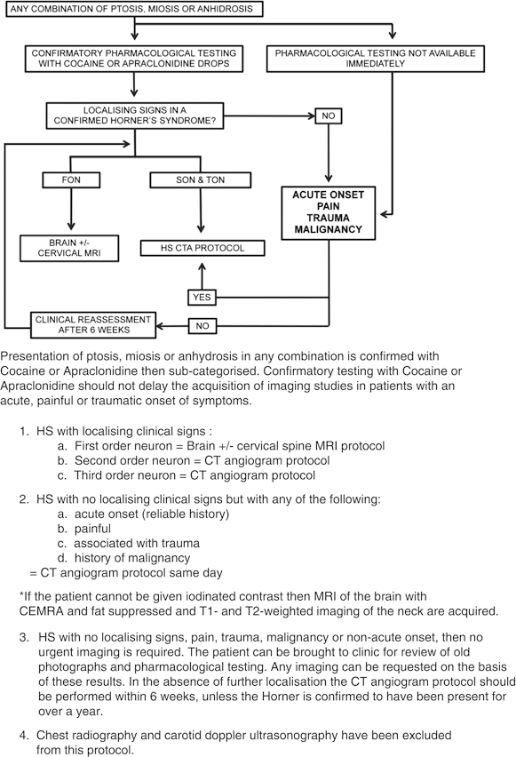
Figure 4. HS Evaluation flowchart (Davagnanam, Eye 2013).
- Testing and subsequent management is greatly influenced by the antecedent history as detailed above.
- Depending on the history presented by the patient and the suspected etiology, imaging and management should not be delayed for pharmacologic diagnosis of HS.
- Imaging protocol is largely based on acuity, presence of pain, and the neuronal order.
- HS patients who present with no localizing signs, pain, or trauma, no history of malignancy, and nonacute onset
- Do not require urgent imaging
- Can be evaluated with pharmacologic testing, reassessment, and subsequent directed imaging if necessary
- In the absence of localization and inability to confirm that HS was present for more than 1 year (through review of photos), proceed with computed tomography angiogram (CTA) of aortic arch, carotid arteries, and intracranial vessels and computed tomography (CT) of the orbits and lung apices.
- HS with acute onset, pain (neck, headache, facial), trauma, or history of malignancy
- CTA of aortic arch, carotid arteries, and intracranial vessels, and CT of the orbits and lung apices
- Imaging on the day of evaluation
- 18% of all dissections result in an ocular or hemispheric stroke over 1 hour to 31 days with a majority occurring in the first 2 weeks (Biousse, Am J Ophthalmol 1998), therefore imaging should not be delayed.
- FON HS or HS with central nervous system signs
- Ataxia, hemiparesis, or upper-limb paresthesiae
- MRI brain
- If there are no brain or brainstem signs, also do MRI of the cervical and thoracic spine.
- SON and TON HS with localizing signs
- CTA of aortic arch, carotid arteries, and intracranial vessels and CT of the orbits and lung apices
Testing to establish diagnosis in children
- Full physical examination, including palpation of neck, axilla, and chest
- Urine testing of catecholamine metabolites homovanillic acid (HVA) and vanillylmandelic acid (VMA) is a common screen for neuroblastoma
- Inadequate as the sole screening method due to the high false- negative rate. Mahoney et al. reported a false negative VMA/HVA in all four patients who were later found to have neuroblastoma (Mahoney, Amer J Ophthal 2006)
- Typically avoid CT scan if possible due to radiation exposure
- MRI head, neck, and chest
- The above screening should be considered for all patients given the rare but reported cases of neuroblastoma in children that would not have normally been screened. A case in the Mahoney et al. study had a history of birth trauma, but was also found to have neuroblastoma on work-up.
Risk factors
- Vascular disorder (stroke, dissection, aneurysm, thrombosis, arteritis, AVM) risk factors, including hypertension, tobacco use, diabetes, heart disease
- Connective tissue disorders
- Nerve disorder (demyelination, myelitis) risk factor: female gender, younger age (20s–40s)
- Lung tumor risk factor: tobacco use
- Trauma risk factors: surgical procedure, accident, forceps delivery, neck manipulation for therapeutic purposes
Differential diagnosis
- Other causes of ptosis
- Pharmacologic pupil
- CN III palsy
- Contralateral iris sphincter tear
- Physiologic anisocoria
- Inflammatory pupil changes i.e. due to uveitis
Patient management: treatment and follow-up
- Address underlying cause of HS.
- Once stable, can repair ptosis, if visually or cosmetically significant
- Muller muscle resection (Glatt, Ophthalmic Surg 1990)
- Amount of resection is determined by phenylephrine testing, as in other forms of blepharoptosis.
- Despite possibility of increased sensitivity to phenylephrine, there were no changes made to the amount of resection and outcomes were consistent with their previous reports.
- Levator aponeurosis advancement
- Levator advancement can be expected to produce satisfactory and consistent correction of ptosis while allowing for easier management of under or overcorrection.
- Internal carotid artery dissection
- Suspected ICAD needs to be managed urgently if clinical signs point to acute onset.
- Management typically involves aspirin, anticoagulation, and/or antiplatelet medications for 3–6 months.
- Additional management can include thrombolytics, vascular stenting, embolization, or other surgical management.
- Risk reduction
- There are no proven methods to reduce risk of recurrence.
- Some suggest patients with dissection avoid contact sports, chiropractic neck manipulation, and any activity that involve abrupt neck movements.
Complications of treatment
- Ptosis repair
- Under- or overcorrection
- Infection
- Dry eyes
- Recurrence
- Scarring, hemorrhage
Disease-related complications
- Sequela associated with vascular disorders, nerve disorders, trauma, tumors
Advances in HS
- Changes in the pharmacologic testing
- Advances in neuroimaging techniques have provided improved detection of lesions
References and additional resources
- AAO, Basic and Clinical Science Course. Section 5: Neuro-Ophthalmology, 2013-2014.
- AAO, Focal Points: Clinical Importance of Pupillary Inequality, Module #10, 1992, p. 2-6, 9-11.
- AAO, Surgery of the Eyelid, Orbit & Lacrimal system, Vol. 2, 1994, p.63, 125-127.
- AAO, Surgical Anatomy of the Ocular Adnexa, 1996, p.24.
- Biousse V1, Touboul PJ, D’Anglejan-Chatillon J, Lévy C, Schaison M, Bousser MG. Ophthalmologic manifestations of internal carotid artery dissection. Am J Ophthalmol. 1998 Oct;126(4):565-77.
- Brazis PW, Masdeu, JC. Localization in Clinical Neurology. Philadelphia, PA: LWW, 2011
- Chang CA et al. Isolated Horner Syndrome from an elongated styloid process (Eagle Syndrome). J Neuroophthalmol 2015 May 20.
- Chen PL1, Chen JT, Lu DW, Chen YC, Hsiao CH. Comparing efficacies of 0.5% apraclonidine with 4% cocaine in the diagnosis of Horner syndrome in pediatric patients. J Ocul Pharmacol Ther. 2006 Jun;22(3):182-7.
- Chen PL1, Hsiao CH, Chen JT, Lu DW, Chen WY. Efficacy of apraclonidine 0.5% in the diagnosis of Horner syndrome in pediatric patients under low or high illumination. Am J Ophthalmol. 2006 Sep;142(3):469-74.
- Danesh-Meyer HV, Savino P, Sergott R. The correlation of phenylephrine 1% with hydroxyamphetamine 1% in Horner’s syndrome. Br J Ophthalmol. 2004 Apr;88(4):592-3.
- Davagnanam I1, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013 Mar;27(3):291-8.
- Glatt HJ1, Putterman AM, Fett DR. Muller’s muscle-conjunctival resection procedure in the treatment of ptosis in Horner’s syndrome. Ophthalmic Surg. 1990 Feb;21(2):93-6.
- Jacobson DM1, Berg R, Grinstead GF, Kruse JR. Duration of positive urine for cocaine metabolite after ophthalmic administration: implications for testing patients with suspected Horner syndrome using ophthalmic cocaine. Am J Ophthalmol. 2001 Jun;131(6):742-7.
- Kardon RH1, Denison CE, Brown CK, Thompson HS. Critical evaluation of the cocaine test in the diagnosis of Horner’s syndrome. Arch Ophthalmol. 1990 Mar;108(3):384-7.
- Kucur C et al. A rare complication of radiofrequency tonsil ablation: horner syndrome. Case Rep Otolaryngol 2015; 2015:570520.
- Lebas M1, Seror J, Debroucker T. Positive apraclonidine test 36 hours after acute onset of horner syndrome in dorsolateral pontomedullary stroke. J Neuroophthalmol. 2010 Mar;30(1):12-7.
- Lyrer PA1 et. al; Cervical Artery Dissection and Ischemic Stroke Patients (CADISP) Study Group.. Clinical import of Horner syndrome in internal carotid and vertebral artery dissection. Neurology 2014 May 6;82(18):1653-9.
- Mahoney NR, Liu GT, Avery RA, Menacker SJ, Wilson MC, Hogarty MD, Maris JM. Pediatric horner syndrome. Arch Ophthalmol. 2011 Aug;129(8):1108-9.
- Maloney WF, Younge BR, Moyer NJ. Evaluation of the causes and accuracy of pharmacologic localization in Horner’s syndrome. Am J Ophthalmol. 1980 Sep;90(3):394-402.
- Martin, TJ, Corbett, JJ. The Pupil. In: Neuro-ophthalmology: the requisites in ophthalmology, Krachmer, JH (Ed), St. Louis, MO: Mosby; 2000.
- Murchison AP1, Rosen MR, Bilyk JR. Horner syndrome as a presenting sign of nasopharyngeal carcinoma. Ophthal Plast Reconstr Surg. 2009 Sep-Oct;25(5):401-2.
- Parwar BL1, Fawzi AA, Arnold AC, Schwartz SD. Horner’s syndrome and dissection of the internal carotid artery after chiropractic manipulation of the neck. Am J Ophthalmol. 2001 Apr;131(4):523-4.
- Rosenberg ML.The friction sweat test as a new method for detecting facial anhidrosis in patients with Horner’s syndrome. Am J Ophthalmol. 1989 Oct 15;108(4):443-7.
- Shah AV, Paul-Oddoye AB, Madill SA, Jeffrey MN, Tappin AD. Horner’s syndrome associated with giant cell arteritis. Eye (Lond). 2007 Jan;21(1):130-1.
- Smit DP. Pharmacologic testing in Horner’s syndrome – a new paradigm. S Afr Med J. 2010 Nov 9;100(11):738-40.
- Horner’s syndrome. Available at: http://www.uptodate.com/contents/horners-syndrome. Accessed March 1, 2014.
- Van der Wiel HL, Van Gijn J. Localization of Horner’s syndrome. Use and limitations of the hydroxyamphetamine test. J Neurol Sci. 1983 May;59(2):229-35.
- Watts P1, Satterfield D, Lim MK. Adverse effects of apraclonidine used in the diagnosis of Horner syndrome in infants. J AAPOS. 2007 Jun;11(3):282-3.
- Weinstein JM, Zweifel TJ, Thompson HS. Congenital Horner’s syndrome. Arch Ophthalmol. 1980 Jun;98(6):1074-8.
- Wessel MM1, Dinkin MJ, Phillips CD, Lelli GJ Jr. Traumatic ptosis and mydriasis masking Horner syndrome from an internal carotid pseudoaneurysm. Ophthal Plast Reconstr Surg. 2011 Jul-Aug;27(4).
