IgG4-Related Disease
Updated May 2025
Establishing the diagnosis
IgG4-related disease (IgG4-RD) is a multi-organ, inflammatory disease characterized by tumefactive lesions with dense lymphoplasmacyctic infiltration, rich in IgG4 positive plasma cells, fibrosis, and in some organs, an obliterative phlebitis. Serum IgG4 levels can be elevated or normal. The orbit is a frequently involved site (IgG4-ROD), and the majority of patients with orbital disease will have disease elsewhere.
Etiology
- Etiology remains unknown.
- Some evidence exists for disease susceptibility in some HLA types, at least in Asian patients.
- There is some (limited) evidence of a role for infection with Helicobacter pylori, at least in patients with autoimmune pancreatitis (AIP). Molecular mimicry in genetically susceptible patients might play a role in pathogenesis.
- The exact role (if any) for IgG4+ plasma cells or IgG4 in the disease process is unknown.
- IgG4 is the fourth subclass of IgG, and normally forms its smallest fraction.
Epidemiology
- Much published literature is from Asia (Japan, Korea, Hong Kong), but there is no evidence that IgG4-RD is more common there.
- Incidence of AIP in Japan is 0.82 per 100,000, and in 95% of cases, onset occurs after age 45.
- AIP (IgG4-related pancreatitis) has a strong male predominance (2.8–7.5:1), the opposite of most auto-immune diseases.
- Head and neck IgG4-RD has a more even male:female ratio.
- 39–48% of patients with AIP will have or develop lacrimal or salivary gland lesions.
- Patients presenting with IgG4-ROD with systemic disease (disease elsewhere) at presentation are reported at 22–100% (the mean is 50%).
- Mean age of onset for IgG4-ROD is 55 years (slightly younger than for AIP, in which the mean is 58–69). In children, there are some rare reported cases.
- 25–40% of cases historically labeled idiopathic orbital inflammation or reactive lymphoid hyperplasia have been shown to be IgG4-ROD.
Clinical features
- Most patients have an indolent, chronic disease without an obvious acute inflammatory process at any time.
- Symptoms of inflammation, such as pain, redness, and swelling, are rarely seen.
- IgG4-ROD can present in many ways, but the most common patterns include:
- Lacrimal gland swelling, usually bilateral, sometimes associated with symmetrically enlarged salivary glands (submandibular and parotid, Mickulicz’s disease, Cases 1, 2, and 3)
- Enlargement of the infraorbital nerve(s) (IONE) or frontal nerve(s) associated with lacrimal gland disease, extraocular muscle disease, patchy orbital fat involvement, paranasal sinusitis, and peripheral eosinophilia (Cases 4, 5, and 6)
- Sclerosing orbital inflammation, sometimes with adjacent lacrimal drainage apparatus and nasal involvement (Case 7)
- Rarely, scleritis and mild anterior uveitis; exceptionally, conjunctival disease associated with scleritis
- Some reports suggest a proportion of periocular xanthogranulomatous disease have numbers of IgG4+ plasma cells to reach IgG4-ROD diagnostic levels, but the relationship between IgG4-ROD and xanthogranulomatous disease remains unknown.
- IONE, unilateral or bilateral, is a strong marker for IgG4-ROD. Sensory loss is rare. Other nerves can be affected, including perioptic nerve lesions. Bony expansion of the infraorbital canal is often seen (Cases 4, 5, and 6).
- Patients can have symptomatic or asymptomatic IgG4-related disease elsewhere, with salivary gland, pancreas, hepatobiliary tree, lymph nodes, retroperitoneum, kidneys, and aorta being most common. However, almost any organ can be affected.
- Disease elsewhere can be present at the same time or develop later, often after many years.
- IgG4-RD appears to predispose to the development of lymphoma, usually low-grade marginal zone lymphoma of the MALT type.
Establishing the diagnosis
- Diagnosis requires integrating clinical features, blood tests, imaging, and histopathology.
- Histopathology is the key to the diagnosis.
- Classic features on histopathology are:
- Lymphoplasmacytic infiltrate
- Fibrosis, classically in a storiform pattern (not usually seen in the lacrimal gland)
- Obliterative phlebitis (more usually seen in pancreas and larger organs, not in lacrimal gland)
- Presence of significant numbers of IgG4+ plasma cells (the numbers required to reach published diagnostic criteria have varied, from minimum 10/high power field up to 100/hpf)
- Ratio of IgG4+ plasma cells to IgG+ plasma cells of at least 40%
- Large numbers of eosinophils
- Use caution in making the diagnosis when features such as granulomatous inflammation, infiltration with polymorphs, and necrosis are present.
- Many other diseases can have significant numbers of IgG4+ plasma cells present. The ratio of IgG4+/IgG+ is probably more important.
- Serum IgG4 elevation can be helpful, but is not diagnostic. Levels above 135 mg/dl are indicative of IgG4-related disease, but on their own, they are not diagnostic.
Disease staging
- To determine whether IgG4-related disease is present in other anatomical sites, the first step is a thorough clinical history and examination. This might be best performed by a specialist such as a rheumatologist.
- Blood tests to look for other organ involvement might include liver function and renal function, and serum IgG4 levels. Use renal ultrasound to exclude obstructive nephropathy from retroperitoneal fibrosis.
- Imaging by CT or MRI from the head and neck to the pelvis should detect other tumefactive lesions, and retroperitoneal disease.
- PET-CT scanning can be very useful in detecting disease activity in all anatomical sites, including the retroperitoneum, aorta, and other difficult to image sites. PET-CT can also be useful in monitoring response to treatment.
Risk factors
- While no definite risk factors have been identified, smoking may be associated with greater odds of having IgG4-RD, especially in women and those with retroperitoneal fibrosis or normal IgG4 concentrations.
Differential diagnosis
- Orbital Inflammatory Diseases including Sarcoidosis, Sjogren Syndrome, Idiopathic Orbital Inflammation, Granulomatosis with Polyangiitis (Wegener), Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss), and Erdheim-Chester Disease.
- Lymphoma
- Reactive lymphoid lesions
- Other causes of unilateral lacrimal gland enlargement, both benign and malignant
Patient management: treatment and follow-up
Natural history
- IgG4-ROD is generally an indolent, chronic process.
- It can progress slowly over many years, and evolve from single organ to multiple organ involvement.
- The disease process can lead to organ damage and loss of function.
- IgG4-ROD can lead to visual loss in a small number of cases, usually by optic nerve compression.
- Some cases can develop lymphoma after many years (Cases 5 and 6).
- Spontaneous resolution has been noted occasionally.
Medical therapy
- Corticosteroids (IV or Oral) are the first step in managing IgG4-RD
- Lack of response should lead one to question the diagnosis of IgG4-RD
- Optimal regimen and duration remain an area of ongoing research
- Initial dose of Prednisone of at least 0.6mg/kg/day, or its equivalent, for 4weeks
- This may be followed by taper of 5mg every 1-2weeks
- There is no consensus on the role of corticosteroids in remission maintenance
- Maintenance dosage of 5mg has been shown to reduce the rate of recurrence in AIP
- Rituximab (anti-CD20) is also an effective treatment option as first-line treatment and at preventing flares
- Other disease modifying and biologic agents, like methotrexate, azathioprine, cyclophosphamide, mycophenolate mofetil have also been used, but the supporting evidence has been limited.
Radiation
- A very small number of cases of IgG4-ROD treated with low-dose radiotherapy have been reported, with variable response.
- The rationale for radiotherapy is based on the response of reactive lymphoid lesions and low-grade lymphomas to this modality.
Surgery
- The main and essential role for surgery is in obtaining biopsy material.
- Rarely bulky nonfunctioning lacrimal glands can be excised.
- Rare cases of optic nerve compromise due to compression that do not respond to medical treatment might benefit from orbital decompression.
Response to treatment and monitoring response
- As already noted, many respond to corticosteroids, but with relapse on tapering or ceasing treatment.
- Nonsteroid immune-suppressants, including Rituximab, offer an alternative.
- Reduction in mass lesions gives a good indication of response in IgG4-ROD.
- Clinical monitoring of orbital disease long-term is advisable because of the risk of relapse or transformation to low-grade lymphoma.
- Other organ involvement should be monitored by the appropriate specialist.
- Serum IgG4 levels can provide an indication of response to treatment.
- PET-CT is a useful means of monitoring response to treatment.
Preventing and managing treatment complications
- Side effects of systemic corticosteroids include glaucoma, cataracts, hyperglycemia, weight gain, mood changes, peripheral edema, systemic hypertension, immunosuppression, osteoporosis, and gastritis. Ongoing monitoring and prevention of medication-related morbidity should be coordinated with the patient’s other medical caregivers.
- Complications of biopsies are minimal. Biopsy of the infraorbital nerve can lead to permanent numbness not present before the biopsy.
Disease-related complications
- Dry eye from lacrimal gland dysfunction
- Ocular motility disturbance from extraocular muscle (EOM) disease
- Rarely, loss of function in affected orbital nerves
- Visual compromise from optic-nerve compression at the orbital apex or perioptic nerve disease (Case 6)
- Corneal exposure related to proptosis
- Lacrimal obstruction and infection if lacrimal drainage apparatus affected
- Loss of function in other affected organs, including renal impairment (obstructive nephropathy from retroperitoneal disease, interstitial nephritis), pancreatic dysfunction, hepatobiliary dysfunction, salivary gland dysfunction, inflammatory aortic aneurysms, and so on
Historical perspective
- Elevated serum IgG4 was first noted in patients with auto-immune pancreatitis (AIP) in 2001, in Japan.
- Soon after, extra-pancreatic fibro-inflammatory lesions were found in other organs, rich in IgG4+ plasma cells, found to be associated with AIP.
- The lacrimal gland is one of the earliest extra-pancreatic sites found to be affected by IgG4-related disease.
- In 2011, comprehensive clinical and pathological diagnostic criteria were published (Umehara et al).
- Consensus statement on the pathology of IgG4-RD was published in 2012 (Deshpande et al).
- Many fibro-inflammatory diseases are now thought to be part of the IgG4-RD spectrum, including Mickulicz disease (symmetrically enlarged lacrimal and salivary glands, Case 2), multifocal fibrosclerosis (sclerosing orbital inflammation with similar lesions in other organs, Cases 3 and 6), eosinophilic angiocentric fibrosis (a form of sclerosing inflammation usually affecting the nose and paranasal sinuses and sometimes the lacrimal drainage apparatus and orbit, Case 7), and many others.
Cases
Case 1
Unilateral sclerosing dacryoadenitis
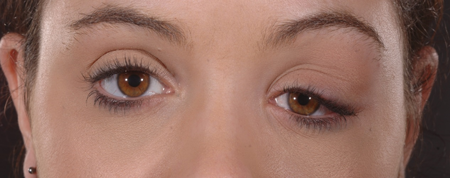
Figure 1-1. A 27-year-old female, otherwise well, presented with 4 weeks increasing left upper lid swelling with mild ache, and a hard tender mass in the left lacrimal gland.
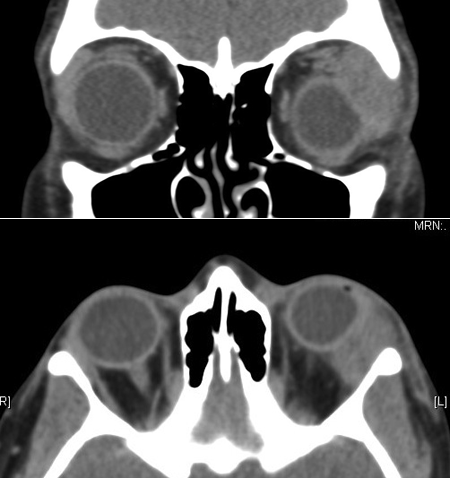
Figure 1-2. CT scans show a grossly enlarged lacrimal gland with flattening of the globe.
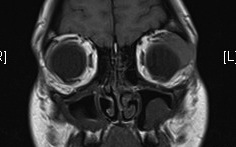
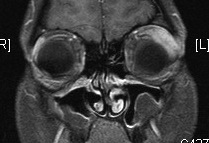
Figure 1-3. MRI shows a discrete lacrimal gland lesion, which enhances with gadolinium.
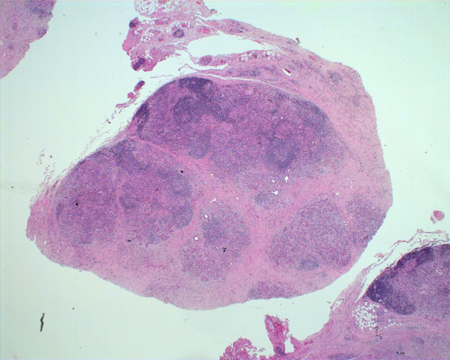
Figure 1-4. Biopsy showed a hard, pale mass in the lacrimal gland, which on H and E section on low power, showed masses of lymphoplasmacytic infiltrate separated by bands of fibrosis.
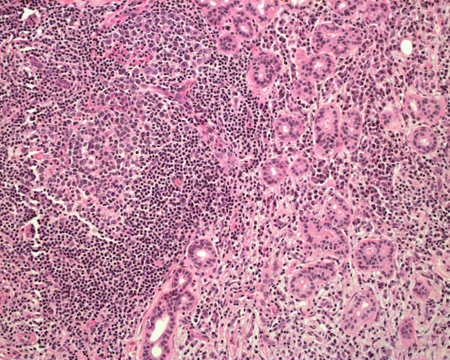
Figure 1-5. On higher power, the lymphoplasmacytic infiltrate also separated portions of relatively normal glandular structures with some reactive follicle formation.
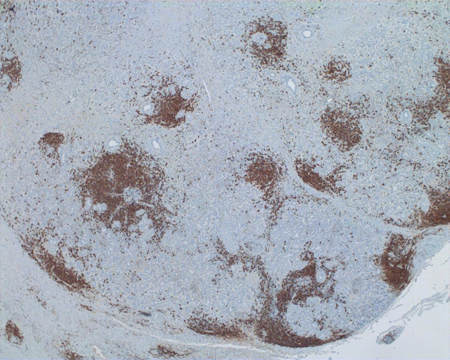
Figure 1-6. Immunohistochemical staining for CD20+ lymphocytes showed many within areas of lymphoplasmacytic infiltrate and follicle formation.

Figure 1-7. Immunostaining for CD3, a T-cell marker, shows numerous T-cells.
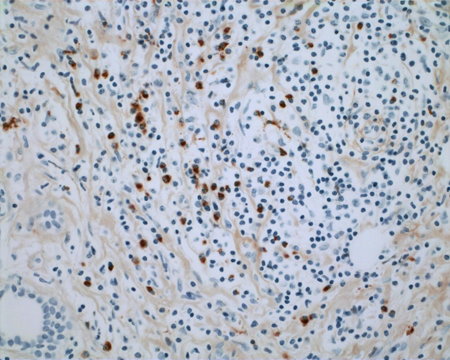
Figure 1-8. Immunostaining for IgG4 shows large numbers of IgG4+ plasma cells up to 96/high power field.
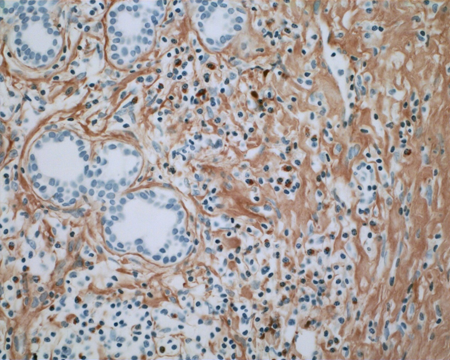
Figure 1-9. Immunostaining for IgG shows smaller numbers of IgG+ plasma cells (but more diffuse background staining, of no significance) and a ratio of IgG4+/IgG+ plasma cells of 137%. Serum IgG4 levels were normal. CT chest, abdomen and pelvis were normal. She responded well to a course of oral steroids.
Case 2
Bilateral lacrimal and salivary gland disease
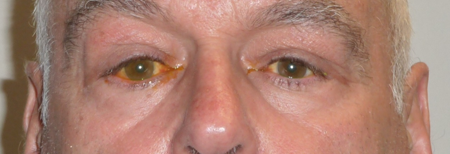
Figure 2-1. A 58-year-old man presented in late 2012. A year before, an enlarged left submandibular gland was removed and diagnosed as bilateral lacrimal and salivary gland disease (formerly known as Mickulicz disease). An enlarged right submandibular gland was removed 3 months before presentation. There was a long history of rhinosinusitis, endoscopic sinus surgery a year earlier, and a 3-year history of asthma. For 9 months he had noted swelling in both upper lids and mild ptosis. The lacrimal glands were palpably enlarged and firm.
Figure 2-2. Both parotid glands were enlarged, the left more than right.
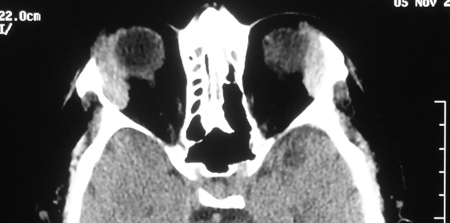
Figure 2-3. An axial CT scan showed bilateral, symmetrically enlarged lacrimal glands. Serum IgG4 levels were markedly elevated, and he had a mild eosinophilia and ESR of 29. Biopsy of one lacrimal gland showed firm, pale tissue, with atrophic gland, dense fibrosis and reactive lymphoid follicles, 90 IgG4+ plasma cells/hpf, and an IgG4+/IgG+ plasma cell ratio of 150%. Review of the salivary glands showed similar pathology. He was started on 40 mg of prednisolone with a good clinical response, but when reduced below 7.5 mg, symptoms recurred. Introducing Azathioprine with low-dose prednisolone got a good and well-maintained response.
Case 3
Bilateral, asymmetric lacrimal gland and orbital fat disease, rhinosinusitis, and sclerosing cholangitis
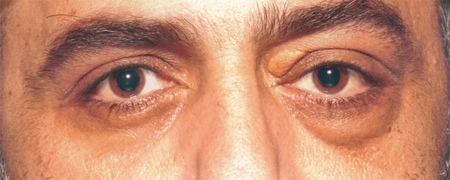
Figure 3-1. A 41-year-old man presented mid 2009 with 5 months of swelling in the left lacrimal gland and chronic rhinosinusitis. He had recently developed grossly deranged liver function tests and was found to have autoimmune sclerosing cholangitis and a grossly elevated serum IgG4 levels.
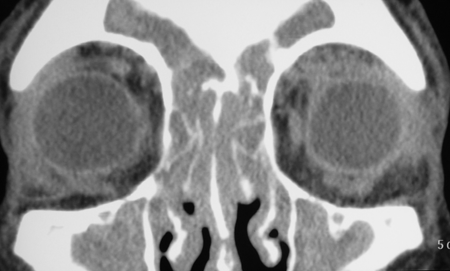
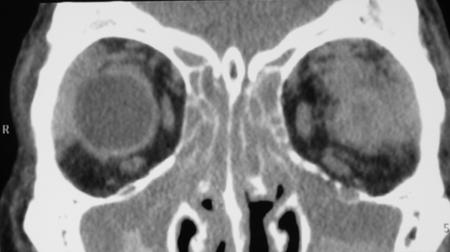
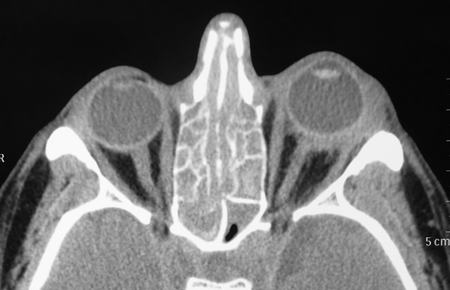
Figure 3-2. Coronal and axial CT scans show pansinusitis and poorly defined enlarged lacrimal glands with an infiltrative process spilling over into the orbital fat. The second coronal CT scan shows possible enlargement of the infraorbital nerve. Biopsy of the left lacrimal gland showed chronic sclerosing dacryoadenitis with large numbers of IgG4+ plasma cells and a ratio of IgG4+/IgG+ plasma cells of 41%. He was treated with oral steroids, with improvement in his orbital and nasal symptoms and normalization of liver function tests, but relapse on tapering of the oral steroids, treated with Azathioprine and low dose steroids.
Case 4
Mickulicz pattern evolved to extraocular muscle disease, enlarged infraorbital nerves, and sinus disease
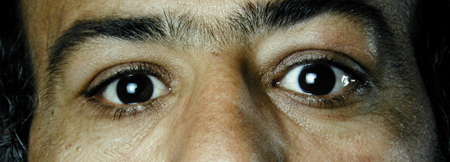
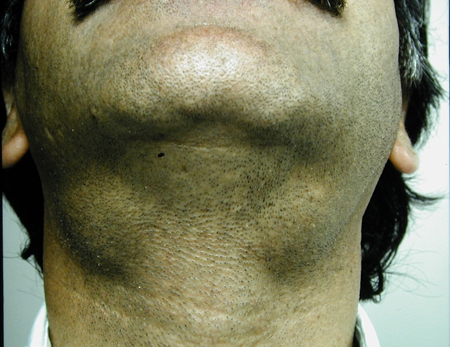
Figure 4-1. A 37-year-old man presented in 1991 with bilateral lacrimal and submandibular gland enlargement.
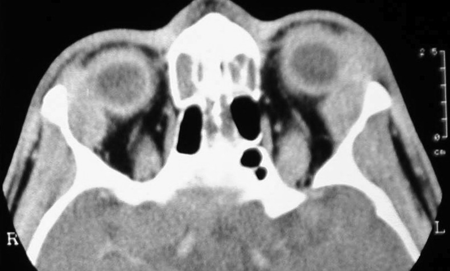
Figure 4-2. An axial CT scan showed the enlarged lacrimal glands, which on biopsy showed reactive lymphoid hyperplasia. He responded to a short course of oral steroids and was lost to follow-up.
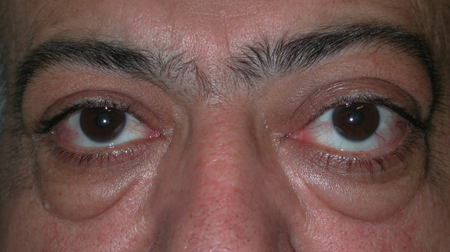
Figure 4-3. In 2001, he returned with 2–3 years of increasing bilateral proptosis and lid swelling.
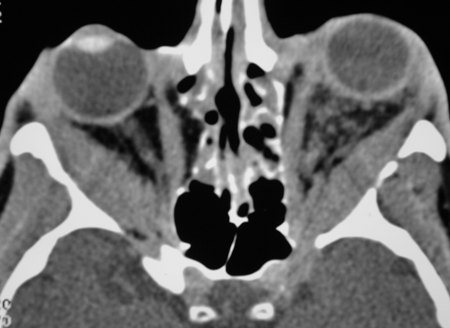
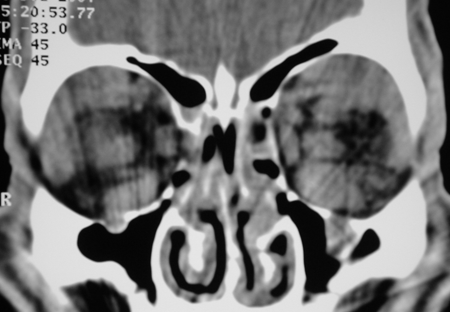
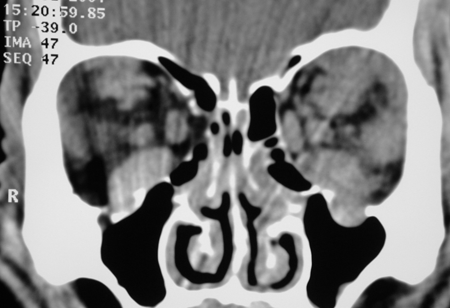
Figure 4-4. CT scans now showed enlarged extraocular muscles, disease in the lacrimal glands and superior orbits, and definite enlargement of the infraorbital nerves and canals. Biopsies from the orbit showed reactive lymphoid hyperplasia again. He responded to oral steroids, but moved overseas and was again lost to follow-up. In 2007 these biopsies were reviewed, and he had IgG4+ plasma cells of 150/hpf and an IgG4+/IgG+ plasma cell ratio of 300%.
Case 5
Lacrimal gland swelling, cervical lymphadenopathy, asthma, chronic rhinosinusitis, enlarged infraorbital nerves, developed diffuse large B-cell lymphoma and died from lymphoma
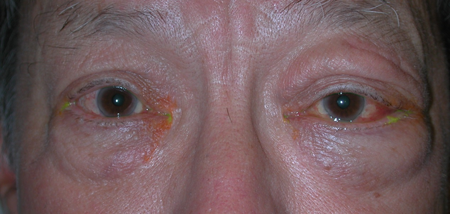
Figure 5-1. A 51-year-old man first presented in 1991 with a year of left lacrimal gland swelling, cervical lymphadenopathy, asthma, chronic sinusitis, and an urticarial skin rash. He had been diagnosed with Sjogren’s disease, but had negative serology. Biopsy of the left lacrimal gland was diagnosed as reactive lymphoid hyperplasia. He responded well to oral steroids and chlorambucil.
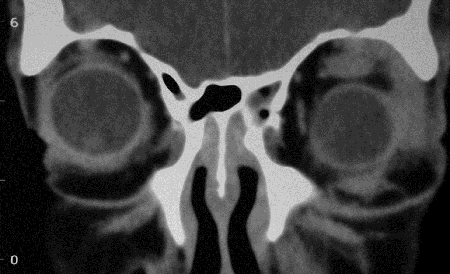
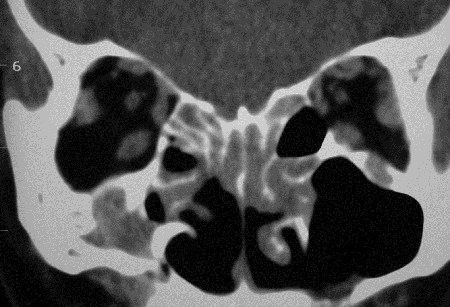
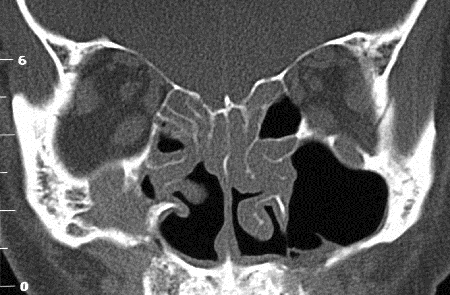
Figure 5-2. CT scans showed lacrimal gland disease with some mild enlargement of some EOMs, rhinosinusitis and changes from previous sinus surgery, and grossly enlarged infraorbital nerves and canals on each side. He returned in 2008 with increasing bilateral proptosis. He was rebiopsied and found to have large numbers of IgG4+ plasma cells. He was treated with oral prednisolone and methotrexate with a good response. Two years later, he developed diffuse large B-cell lymphoma, and 18 months later, died from his disease.
Case 6
Enlarged lacrimal glands and frontal and infraorbital nerves, cervical lymphadenopathy, enlarged parotid glands and submandibular glands, primary biliary cirrhosis, developed left perioptic nerve disease, on rebiopsy, marginal zone lymphoma of MALT type
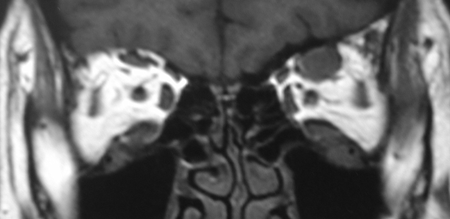
Figure 6-1. A 52-year-old woman presented in 2008 with a 1-year history of bilateral lacrimal gland enlargement, cervical lymphadenopathy, and salivary gland enlargement. She had a history of primary biliary cirrhosis, and atypical Sjogren’s syndrome with negative serology. MRI scans showed an enlarged left frontal nerve, which was biopsied and showed a dense lymphoplasmacytic infiltrate and features of IgG4-RD.
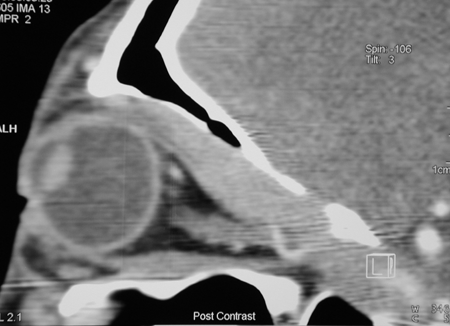
Figure 6-2. A sagittal CT scan showed the frontal nerve enlargement extending through the superior orbital fissure into the cavernous sinus. The left lacrimal gland and frontal nerve were biopsied. They showed reactive lymphoid hyperplasia with large numbers of IgG4+ plasma cells, a ratio of IgG4+/IgG+ plasma cells of 80%, and a markedly elevated serum IgG4 level. She was treated with low-dose prednisolone and hydroxychloroquine by her rheumatologist with minimal response.
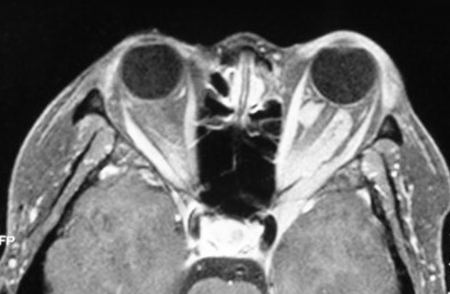
Figure 6-3. Two years later, she developed worsening vision in her left eye, and a repeat MRI showed disease surrounding the optic nerve and extending through the superior orbital fissure into the cavernous sinus.
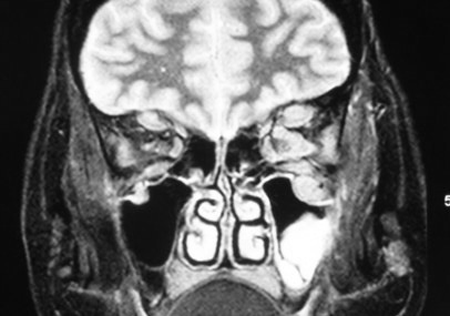
Figure 6-4. A coronal MRI showed more enlargement of the left frontal nerves than of the right, and enlargement of the left infraorbital nerve and canal. A rebiopsy showed transformation to marginal zone lymphoma of MALT type. She was treated with low-dose orbital irradiation with improvement in vision and proptosis.
Case 7
Sclerosing orbital, lacrimal system and nasal disease, formerly known as Eosinophilic Angiocentric Fibrosis
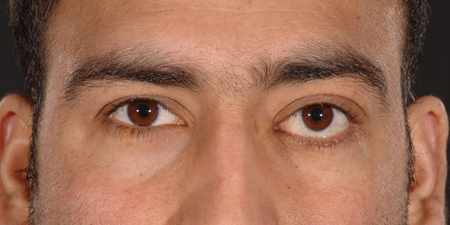
Figure 7-1. A 25-year-old man presented in 2008 with 6 months of left epiphora and proptosis, with a lacrimal mucocele. There was a hard inferomedial orbital mass.
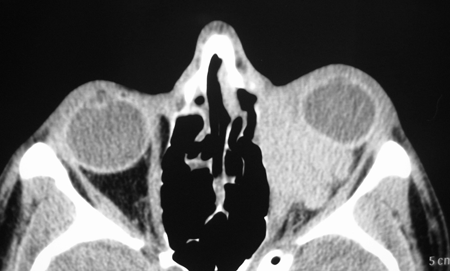
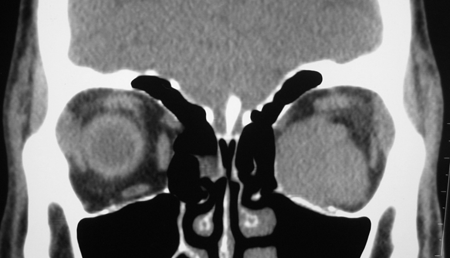
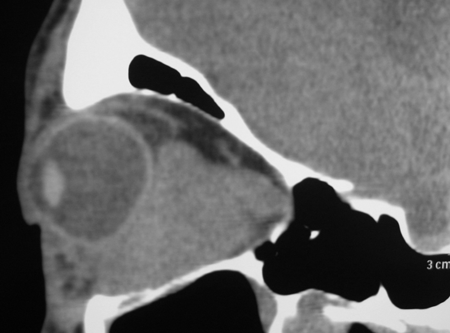
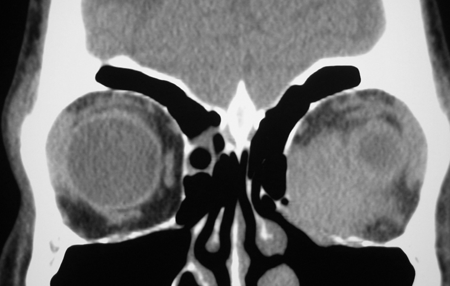
Figure 7-2. CT scans showed a large dense mass involving the inferomedial orbit compressing and displacing the globe. A biopsy diagnosed eosinophilic angiocentric fibrosis. He then underwent a DCR with repeat biopsy of the orbit and thickened nasal mucosa. There was dense fibrosis with large numbers of IgG4+ plasma cells and a mild increase in serum IgG4. He was commenced on oral steroids and methotrexate with stabilization of disease. Rituximab is being considered if his disease worsens.
References and additional resources
General and Reviews
- Carruthers MN, Stone JH, Khosroshahi A. The latest on IgG4-RD: a rapidly emerging disease. Curr Opin Rheumatol 2012;24:60-69
- Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2014 Mar 20. doi 10.1136/annrheumdis-2013-204907 [EPub ahead of print]
- Chang SY, Keogh KA, Lewis JE, et al. IgG4-positive plasma cells in granulomatosis with polyangiitis (Wegener’s): a clinicopathological and immunohistochemical study on 43 granulomatosis with polyangiitis and 20 control cases. Hum Pathol 2013;44:2432-7
- Deshpande V, Zen Y, Chan JKC, et al. Consensus statement on the pathology of IgG4-related disease. Hum Pathol 2012;25:1181-92
- Deshpande V. The pathology of IgG4-related disease: criticial issues and challenges. Sem Diag Pathol 2012;29:191-6
- Ebbo M, Grados A, Bernit E, et al. Pathologies associated with serum IgG4 elevation. Int J Rheumatol 2012:602809. doi: 10.1155/2012/602809. Epub 2012 Aug 26
- Ferry JA, Deshpande V. IgG4-related disease in the head and neck. Sem Diagn Pathol 2012;29:235-44
- Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344:732-8
- Kamisawa T, Egawa N, Nakajima H. Autoimmune pancreatitis is a systemic autoimmune disease. Am J Gastroenterol 2003;98:2811-2
- Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stne JH. IgG4-related disease. Annu Rev Pathol Mech Dis 2014;9:315-47
- Masaki Y, Kurose N, Umehara H. IgG4-related disease: a novel lymphoproliferative disorder discovered and established in Japan in the 21st J CLin Exp Hematopathol 2011;51:13-20
- Masaki Y, Dong L, Kurose N, et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis 2009;68:1310-5
- Stone JH, Khosroshahi A, Deshpande V, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum 2012;64:3061-7
- Strehl J, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localized non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol 2011;64:237-43
- Takuma K, Kamisawa T, Anjiiki H, Egawa N, Igarashi Y. Metachronous extrapancreatic lesions in autoimmune pancreatitis. Intern Med 2010;49:529-33
- Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD). 2011. Mod Rheumatol 2012;22:21-30
- Zen Y, Nakanuma Y. IgG4-related disease. A cross-sectional study of 114 cases. Am J Surg Pathol 2010;34:1812-9
Epidemiology
- Deschamps R, Deschamps L, Depaz R, et al. High prevalence of IgG4-related lymphoplasmacytic infiltrative disorder in 25 patients with orbital inflammation: a retrospective case series. Br J Ophthalmol 2013;97:999-1004
- Fujinaga Y, Kadoya M, Kawa S, et al. Characterisitc findings in images of extra-pancreatic lesions associated with autoimmune pancreatitis. Eur J Radiol 2010;76:228-38
- Hamano H, Arakura N, Muraki T, Ozaki Y, Kiyosawa K, Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol 2006;41:1197-2005
- Japanese Study Group of IgG4-related ophthalmic disease. A prevalence study of IgG4-related ophthalmic disease in Japan. Jpn J Ophthalmol 2013;57:573-9
- Kim KP, Kim MH, Song MH, Lee SS, Seo DW, Lee SK. Autoimmune chronic pancreatitis. Am J Gastroenterol 2004;99:1605-16
- Khosroshahi A, Stone JH. A clinical overview of IgG4-related systemic disease. Curr Opin Rheumatol 2011;23:57-66
- Nishimori I, Tamakoshi A, Otsuki M. Prevalence of autoimmune pancreatitis in Japan from a nationwide survey in 2002. J Gastroenterol 2007; Suppl 18:6-8
Eponymous and Other Diseases Subsumed by IgG4-RD
- Aylward GW, Sullivan TJ, Garner A, Moseley I, Wright JE. Orbital involvement in multifocal fibrosclerosis. Br J Ophthalmol 1995;79:246-9
- Dahlgren M, Khosroshahi A, Nielsen P, et al Riedel’s thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res 2013;62:1312-8
- Deshpande V, Khosroshahi A, Nielsen GP, et al. Eosinophilic angiocentric fibrosis is a form of IgG4-related disease. Am J Surg Patol 2011;35:701-6
- Geyer JT, Ferry JA, Harris NL, et al. Chronic sclerosing sialadenitis (Küttner tumor) is an IgG4-associated disease. Am J Surg Pathol 2010;34:202-10
- Karligkiotis A, Volpi L, Ferreli F, et al. Primary orbital eosinophilic angiocentric fibrosis with intranasal extension. Head Neck 2014;36:e8-11
- Khosroshahi A, Carruthers MN, Stone JH, et al. Rethinking “Ormond’s” disease: “idiopathic” retroperitoneal fibrosis in the era of IgG4-related disease. Medicine (Baltimore) 2013;Feb 22 [Epub ahead of print]
- Kitagawa S, Zen Y, Harada K, et al. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Küttner’s tumor). Am J Surg Pathol 2005;29:783-91
- Leibovitch I, James CL, Wormald PJ, Selva D. Orbital eosinophilic angiocentric fibrosis: case report and review of the literature. Ophthalmology 2006;113:148-52
- Stone JR. Aortitis, periaortitis, and retroperitoneal fibrosis, as manifestations of IgG4-related systemic disease. Curr Opin Rheumatol 2011;23:88-94
- Valenzuela AA, Whitehead KJ, Brown I, Sullivan TJ. Eosinophilic angiocentric fibrosis: an unusual entity producing complete lacrimal obstruction. Orbit 2006;25:159-61
- Winn BJ, Rootman J. Sclerosing orbital inflammation and systemic disease. Ophthal Plast Reconstr Surg 2012;28:107-18
- Yamamoto M, Ohara M, Suzuki C, et al. Elevated IgG4 concentrations in serum of patients with Mickulicz’s disease. Scand J Rheumatol 2004;33:432-3
Ocular Adnexal Disease
- Andrew N, Kearney D. Selva D. IgG4-related orbital disease: a meta-analysis and review. Acta Ophthalmol 2013;91:694-700
- Andrew N, Sladden N, Kearney D, Selva. Retrospective IgG4 and IgG staining of non-specific orbital inflammations reveals a significant rate of IgG4-related disease. (submitted for publication)
- Berry-Brincat A, Rose GE. Idiopathic orbital inflammation: a new dimension with the discovery of immunoglobulin G4-related disease. Curr Opin Ophthalmol 2012;23:415-9
- Go H, Kim JE, Kim YA, et al. Ocular adnexal IgG4-related disease: comparative analysis with mucosa-associated lymphoid tissue lymphoma and other chronic inflammatory conditions. Histopathology 2012;60:296-312
- Hagiya C, Tsuboi H, Yokosawa M, et al. Clinicopathological features of IgG4-related disease complicated with orbital involvement. Mod Rheumatol 2013 Oct 21
- Kalapesi FB, Manasses D, Pawade J, Garrott HM, Herbert HM. IgG4-related disease of the orbit, a 10 year retrospective analysis of lacrimal and other orbital biopsies. (Submitted for publication)
- Karamchandani JR, Younes SF, Wamke RA, et al. IgG4-related systemic sclerosing disease of the ocular adnexa: a potential mimic of ocular lymphoma. Am J Clin Pathol 2012;137:699-711
- Kubota T, Moritani S, Katayama M, Terasaki H. Ocular adnexal IgG4-related lymphoplasmacytic infiltrative disorder. Arch Ophthalmol 2010;128:577-84
- Lindfield D, Attfield K, McElvanney A. Systemic immunoglobulin G4 (IgG4) disease and idiopathic orbital inflammation; removing ‘idiopathic’ from the nomenclature? Eye 2012;26:623-9
- Matsuo T, Ichimra K, Sato Y, et al. Immunoglobulin G4 (IgG4)-positive or –negative ocular adnexal benign lymphoid lesions in relation to systemic involvement. J Clin Exp Hematopathol 2010;50:129-42
- Mehta M, Jakobiec F, Fay A. Idiopathic fibroinflammatory disease of the face, eyelids, and periorbital membrane with immunoglobulin G4-postive cells. Arch Pathol Lab Med 2009;133:1251-5
- Plaza JA, Garrity JA, Dogan A, et al. Orbital inflammation with IgG4-positive plasma cells. Manifestation of IgG4 systemic disease. Arch Ophthalmol 2011;129:421-8
- Sato Y, Ohshima K, Ichimura K, et al. Ocular adnexal IgG4-related disease has uniform clinicopathology. Pathol Int 2008;58:465-70
- Sogabe Y, Ohshima K, Azumi A, et al. Location and frequency of lesions in patients with IgG4-related ophthalmic diseases. Graefes Arch Clin Exp Ophthalmol 2014, Jan 3, epub ahead of print
- Takahira M, Ozawa Y, Kawano M, et al. Clinical aspects of IgG4-related orbital inflammation in a case series of ocular adnexal lymphoproliferative disorders. Int J Rheumatol 2012;doi:10.1155/2012/635473
- Wallace ZS, Khosroshahi A, Jakobiec FA, et al. IgG4-related systemic disease as a cause of “idiopathic” orbital inflammation, including orbital myositis, and trigeminal nerve involvement. Surv Ophthalmol 2012;57:26-33
Dacryoadenitis
- Andrew N, Kearney D, Selva D. Applying the consensus statement on the pathology of IgG4-related disease to lacrimal gland lesions. Mod Pathol 2013;26:1150-1
- Cheuk W, Yuen HKL, Chan JKC. Chronic sclerosing dacryoadenitis: part of the spectrum of IgG4-related sclerosing disease? Am J Surg Pathol 2007;31:643-5
- Jakobiec FA, Stacy RC, Mehta M, Fay A. IgG4-positive dacryoadenitis and Küttner submandibular sclerosing inflammatory tumor. Arch Ophthalmol 2010;128:942-4
- Kamisawa T, Takuma K, Kuruma S, et al. Lacrimal gland function in autoimmune pancreatitis. Intern Med 2009;48:939-43
- Koizumi S, Kamisawa T, Kuruma S, et al. Clinical features of IgG4-related dacryoadenitis. Graefes Arch Clin Exp Ophthalmol 2013; Dec 7
- Lee FJ, Varikatt W, Kairatis K, D’Souza F, Lin MW. IgG4-related dacryoadenitis. Ophthalmology 2010;117:398 e-3-4
- Takahira M, Kawano M, Zen Y, et al. IgG4-related chronic sclerosing dacryoadenitis. Arch Ophthalmol 2007;125:1575-8
Lacrimal Drainage Apparatus IgG4-RD
- Batra R, HS, Sandramouli S. A unique case of IgG4 sclerosing dacryocystitis. Ophthalm Plast Reconstr Surg 2011;17:207-10
- Suzuki M, Mizumachi T, Morita S, Kubota K, Iizuka K. A case of immunoglobulin 4-related disease with bilateral mass forming lesion in the nasolacrimal ducts. J Clin Rheumatol 2011;17:207-10
Conjunctival and Scleral Disease
- Kim EC, Lee SJ, Hwang HS, Kim J, Kim MS. Bilateral diffuse scleritis as a first manifestation of immunoglobulin G4-related sclerosing pachymeningitis. Can J Ophthalmol 2013;48:e31-3
- Ohno K, Sato Y, Ohshima K, et al. IgG4-related disease involving the sclera. Mod Rheumatol 2014;24:195-8
- Paulus YM, Cockerham KP, Cockerham GC, Gratzinger D. IgG4-positive sclerosing orbital inflammation involving the conjunctiva: a case report. Ocul Immunol Inflamm 2012;20:375-7
Pediatric IgG4-RD
- Griepentrog GJ, Vickers RW, Karesh JW, Azari AA, Albert DM, Bukat CN. A clinicopathological case study of two patients with pediatric orbtal IgG4-related disease. Orbit 2013;32:389-91
- Kalapesi FB, Garrott HM, Moldovan C, Williams M, Ramanan A, Herbert HM. IgG4 orbital inflammation in a 5-year-old child presenting as an orbital mass. Orbit 2013;32:137-40
- Sane M, Chelnis J, Kozielski R, Fasiuddin A. Immunoglobulin G4-sclerosing disease with orbital involvement in a 12-year-old girl. J AAPOS 2013;17:548-50
Xanthogranulomatous Disease
- Mudhar HS, Bhatt R, Sandramouli S. Xanthogranulomatous variant of immunoglobulin G4 sclerosing disease presenting as ptosis, proptosis and eyelid skin plaques. Int Ophthalmol 2011;31:245-8
- Roggin KK, Adult-onset asthma and periocular xanthogranulomas in a patient with lymphoplasmacytic sclerosing pancreatitis. Pancreas 2007;34:157-60
- Satchi K, McNab AA, Godfrey T, Prince HM. Adult orbital xanthogranuloma successfully treated with rituximab. Ophthalmology 2014 (in press)
- Singh K, Rajan KDA, Eberhart C. Orbital necrobiotic xanthogranuloma associated with systemic IgG4 disease. Ocular Immunol Inflamm 2010;18:373-8
- Verdijk RM, Heidari P, Verschooten R, van Daele PL, Simonsz HJ, Paridaens D. Raised numbers of IgG4-positive plasma cells are a common histopathological finding in orbital xanthogranulomatous disease. Orbit 2014;33:17-22
Perineural Disease
- Hardy TG, McNab AA, Rose GE. Enlargement of the infraorbital nerve: an important sign associated with orbital reactive lymphoid hyperplasia or IgG4-related inflammation. Ophthalmology 2014 [in press]
- Inoue D, Zen Y, Sato Y, et al. IgG4-related perineural disease. Int J Rheumatol 2012;2012:410890
- Katsura M, Morita A, Horiuchi H, Ohtomo K, Machida T. IgG4-related inflammatory pseudotumor of the trigeminal nerve: another component of IgG4-related sclerosing disease? AJNR 2011;32:E150-2
- Ohshima K, Sogabe Y, Sato Y. The usefulness of infraorbital nerve enlargement on MRI imaging in clinical diagnosis of IGG4-related orbital disease. Jap J Ophthalmol 2012;56:380-2
- Siqueira GB, Jain A, Chahud F, Cruz AA. Bilateral infraorbital nerve involvement in idiopathic orbital myositis. Ophthal Plast Reconstr Surg 2002;18:474-8
- Takano K, Yajima R, Seki N, et al. A study of infraorbital nerve swelling associated with immunoglobulin G4 Mickulicz’s disease. Mod Rheumatol 2013 Dec 29
- Sogabe Y, Miyatani K, Goto R, et al. Pathological findings of infraorbital nerve enlargement in IgG4-related ophthalmic disease. Jpn J Ophthalmol 2012;56:511-4
- Toyoda K, Oba H, Kutomi K, et al. MR imaging if IGG4-related diseasein the head and neck and brain. AJNR 2012;33:2136-9
- Watanabe T, Fujinaga Y, Kawakami S, et al. Infraorbital nerve swelling associated with autoimmune pancreatitis. Jpn J Radiol 2011;29:194-201
- Yokoi S, Kawagashira Y, Ohyama K, et al. Mononeuritis multiplex with tumefactive cellular infiltration in a patient with reactive lymphoid hyperplasia with increased immunoglobulin G4-postive cells. Hum Pathol 2014;45:427-30
IgG4-RD and Lymphoma
- Cheuk W, Yuen HKL, Chan ACL, et al. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: a previously undescribed complication of IgG4-related sclerosing disease. Am J Surg Pathol 2008;32:1159-67
- Matsuo T, Ichimura K, Yoshino T. Local recurrence as immunoglobulinG4 (IgG4)-related disease 10 years after radiotherapy to ocular adnexal extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. J Clin Exp Hematopathol 2011;51:125-33
- Oyama T, Takizawa J, Nakamura N, Aoki S, AizawaY, Abe H. Multifocal mucosa-associated lymphoid tissue lymphoma associated with IgG4-related disease: a case report. Jpn J Ophthalmol 2011;55:304-6
- Sato Y, Ohshima K, Takata K, et al. Ocular adnexal IgG4-producing mucosa-associated lymphoid tissue lymphoma mimicking IgG4-related disease. J Clin Exp Hematopathol 2012;52:51-5
- Sato Y, Takata K, Ichimura K, et al. IgG4-producing marginal zone B-cell lymphoma. Int J Hematol 2008;88:428-33
- Takahashi N, Ghazale AH, Smyrk TC, Mandrekar JN, Chari ST. Possible association between IgG4-associate systemic disease with or without autoimmune pancreatitis and non-Hodgkin lymphoma. Pancreas 2009;38:523-6
- Yamamoto M, Takahashi H, Tabeya T, et al. Risk of malignancies in IgG4-related disease. Mod Rheumatol 2012;22:414-8
Pathogenesis
- Kountouras J, Zavos C, Chatzopoulos D. A concept on the role of Heliocbacter pylori infection in automimmune pancreatitis. J Cell Mol Med 2005;9:196-207
- Zen Y, Nakanuma Y. Pathogenesis of IgG4-related disease. Curr Opin Rheumatol 2011;23:114-8
Imaging
- Ginat DT, Freitag SK, Kieff D, et al. Radiographic patterns of orbital involvement in IgG4-related disease. Ophthal Plast Reconstr Surg 2013;29:261-6
- Matsubayashi H, Furukawa H, Maeda A, et al. Usefulness of positron emission tomography in the evaluation of distribution and activity of systemic lesions associated with autoimmune pancreatitis. Pancreatology 2009; 9:694-9
- Nakajo M, Jinnouchi S, Fukukura Y, Tanabe H, Tateno R, Nakajo M. The efficacy of whole-body FDG-PET or PET/CT for autoimmune pancreatitis and associated extrapancreatic autoimmune lesions. Eur J Nucl Med Mol Imaging 2007; 34:2088-95
- Nguyen VX, De Petris G, Nguyen BD. Usefulness of PET/CT scanning in systemic IgG4-related sclerosing disease. J Pancreas (Online) 2011; 12:297-305
- Song YS, Choung HK, Park SW, Kim JH, Khwang SI, Jeon YK. Ocular adnexal IgG4-related disease: CT and MRI findings. Br J Ophthalmol 2013;97:412-8
- Toyoda K, Oba H, Kutomi K, et al. MR imaging of IgG4-related disease in the head and neck and brain. AJNR 2012;33:2136-9
Treatment
- Carruthers MN, Stone JH, Deshpande V, Khosroshahi A. Development of an IgG4-RD responder index. Int J Rheumatol 2012; Epub 2012 Apr 24
- Detiger SE, Karim AF, Verdijk RM, et al. Acta Ophthalmologica, 97: 451-459, 2019
- Kase S, Yamamoto T, Ishijima K, et al. Spontaneous regression of IgG4-related dacryoadenitis. Mod Rheumatol 2013;23:18-21
- Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum 2010;62:1755-62
- Khosroshahi A, Carruthers MN, Deshpande V, et al. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore) 2012;91:57-66
- Khosroshahi A, Stone JH. Treatment approaches to IgG4-related systemic disease. Curr Opin Rheumatol 2011;23:67-71
- Lindfield D. Rituximab in IgG4-related inflammatory disease of the orbit and ocular adnexae. Eye 2012;26:1386
- Tomiyama T, Uchida K, Matsushita M, et al. Comparison of steroid pulse therapy and conventional oral steroid therapy as initial treatment for autoimmune pancreatitis. J Gastroenterol 2011;46:696-704
Financial disclosures
Reviewers
Viraj Mehta – No disclosures
