Idiopathic Intracranial Hypertension
Updated July 2024
Vivek R. Patel, MD
Establishing the diagnosis
Patients with idiopathic intracranial hypertension (IIH), also known as pseudotumor cerebri (PTC), often present to the ophthalmologist because of complaint of blurred vision or headache. The prompt and accurate recognition of this condition is beneficial both because it establishes the correct diagnosis with a minimum of delay and because IIH is a potentially blinding disorder. Recent advances in the study of the basic biology and the clinical pathophysiology of IIH have changed the approach to this disease condition. IIH is a clinical syndrome characterized by well-defined criteria, originally proposed by neurosurgeon Walter Dandy, MD. These criteria have undergone numerous modifications over the years as our understanding of the condition has improved. The most recent diagnostic requirements are as follows (Friedman et al. Neurology 2013; 81:1159-65).
Diagnostic criteria for pseudotumor cerebri syndrome
- Required for diagnosis of pseudotumor cerebri syndrome
- Papilledema
- Normal neurologic examination except for cranial nerve abnormalities
- Neuroimaging: Normal brain parenchyma without evidence of hydrocephalus, mass, or structural lesion and no abnormal meningeal enhancement on MRI, with and without gadolinium, for typical patients (female and obese), and MRI, with and without gadolinium, and magnetic resonance venography for others; if MRI is unavailable or contraindicated, contrast-enhanced CT may be used
- Normal CSF composition
- Elevated lumbar puncture opening pressure (≥ 250 mm CSF in adults and ≥ 280 mm CSF in children [250 mm CSF if the child is not sedated and not obese]) in a properly performed lumbar puncture
- Diagnosis of pseudotumor cerebri syndrome without papilledema
In the absence of papilledema, a diagnosis of pseudotumor cerebri syndrome can be made if B–E from above are satisfied, and in addition the patient has a unilateral or bilateral abducens nerve palsy
In the absence of papilledema or sixth nerve palsy, a diagnosis of pseudotumor cerebri syndrome can be suggested but not made if B–E from above are satisfied, and in addition at least 3 of the following neuroimaging criteria are satisfied:
- Empty sella
- Flattening of the posterior aspect of the globe
- Distention of the perioptic subarachnoid space with or without a tortuous optic nerve
- Transverse venous sinus stenosis
Epidemiology and etiology
The major risk factors for developing IIH remain obesity and female gender; although no new major epidemiological studies have been reported in the past decade, clinical experience supports their conclusions. The incidence of IIH has increased dramatically despite the current “epidemic of obesity,” and clearly, all obese patients do not develop the condition. Overall the incidence is about 0.7/100,000; however in at-risk populations (e.g., overweight females 15–44 years old) the incidence has been documented to be as high as 19.3/100,000. IIH is virtually nonexistent in countries where obesity is uncommon. In the US, Black and Hispanic patients have a higher prevalence odd ratio of IIH compared with White female patients.
IIH in children (prepubertal) occurs with equal incidence in boys and girls and often follows middle ear infections/mastoiditis, or medication (antibiotic) therapy. Venous sinus thrombosis is not uncommon; most think that pediatric IIH is probably a somewhat different disease than the adult form.
Anatomic factors that increase venous pressure through resistance in the intrathoracic, intra-abdominal, or cerebral intrasinus venous compartments might play a role in causing disease. Regardless of underlying circumstances, the common element is believed be an imbalance between production (usually normal) and absorption of CSF (usually diminished), leading to an elevation of intracranial pressure. Peripheral conversion of estrogen occurs in adipose cells (hence the connection with obesity in women), leading to an increase in circulating levels of the hormone, which in turn affects the capacity of the arachnoid granulations to absorb CSF. Over-excitation of retinoic acid receptors located on the arachnoid granulations can impair CSF absorption (see risk factors below).
Studies over the past decade have provided mounting evidence implicating a role for abnormalities in cerebral venous sinus structure in patients with IIH. It has been recognized that a high proportion (>90%) of patients with IIH have uni- or bilateral transverse sinus (TS) stenosis (Farb et al. 2003, Higgins et al. 2004). However, here is significant debate about whether these sinovenous stenoses play a role in the pathophysiology of this condition or are simply a consequence of raised intracranial pressure (Rohr et al. 2007, Higgins et al. 2004, King et al. 2002, Baryshnik et al. 2004, De Simone et al. 2005, McGonigal et al. 2004). Restoring the patency of the stenotic venous sinus with stenting can normalize intracranial pressure and significantly improve or render some patients asymptomatic (Higgins et al. 2002 and 2003, Owler et al. 2003, Ogunbo et al. 2003, Rajpal et al. 2005, Metellus et al. 2005 and 2007, Rohr et al. 2007, Donnet et al. 2008, Paquet et al. 2008, Bussiere et al 2010).
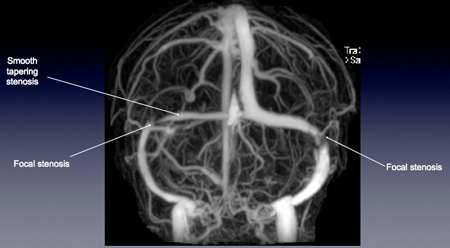
Figure 1. Contrast-enhanced MRV brain in a patient with IIH. In addition to the smooth tapering stenosis noted along the right transverse sinus, the characteristic, reproducible abnormality seen a large majority of IIH patients is unilateral or bilateral focal stenosis at the junction of the distal 1/3 portion of the transverse sinus and sigmoid sinus.
History
In addition to demographic considerations described earlier, patients with IIH typically present with signs and/or symptoms of raised ICP. These can include headache (worse upon awakening or when supine), nausea, pulsatile tinnitus (“whooshing” sounds timed to heartbeat), transient obscurations of vision (momentary greying out of vision or positive visual phenomena (seconds)) occurring spontaneously or with change in postural/valsalva, or binocular (usually) horizontal diplopia due to abducens nerve compression the ventral pontine surface and bony clivus.
Clinical features
Patients with disc swelling due to raised ICP (papilledema) usually present with normal (or near normal) visual acuity, normal color vision, and equal pupillary responses. Indeed the most common clinical finding in IIH is papilledema. Swelling of the retinal nerve fiber layer (RNFL) leads to a loss of transparency of this layer, thereby obscuring visualization of underlying anatomic structures such as the disc margin, blood vessels, and eventually the optic cup.
The severity of papilledema (see Frisen grading system, Figure 2) does not necessarily correlate with the degree of visual loss at the time of presentation. Indeed some patients have advanced VF loss with fairly minimal papilledema, while others have severe disc edema and early VF changes only. This is the most widely used grading system, allowing accurate comparisons over time and across observers.
Symptoms can begin gradually over several weeks to months, or more abruptly with subacute onset of symptoms. Some patients have advanced visual field loss at the time of presentation, suggesting chronicity and ongoing damage over several months to years.
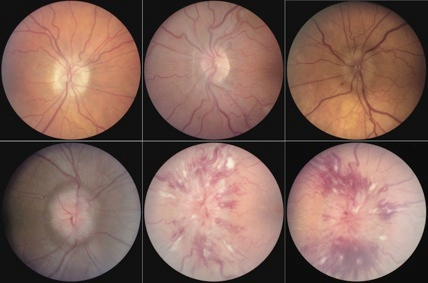
Figure 2. Frisen papilledema grading scale from grade 0 (top left) to grade 5 (bottom right). Recording the papilledema grade is a simple way to track the clinical status. Key features include C-shaped halo of edema in grade 1 (top center), 360-degree edema in grade 2 (top right), and progressive obscuration of vessels at the disc margin (grade 3), on the disc surface centrally (grade 4), and complete obliteration on the disc as well as extensive involvement of peripapillary retina (grade 5). Note hemorrhage and exudate are not part of the grading scheme. The presence of exudation usually indicates a more fulminant, rapid onset development of raised intracranial pressure, overwhelming the retinal vasculature’s ability to compensate.
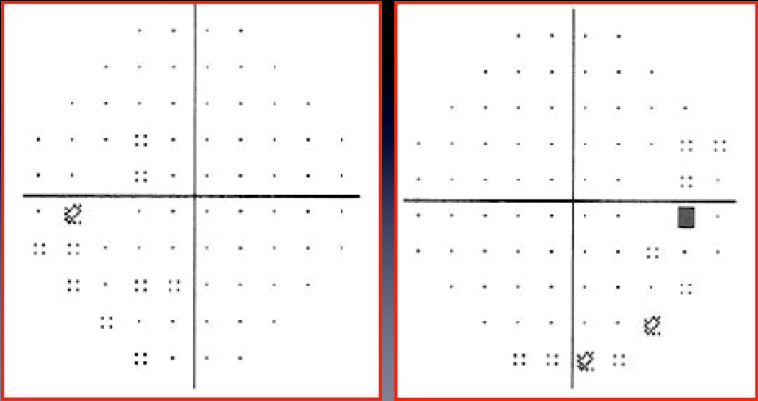

Figure 3. HVF in patients with IIH. Left eye on the left, right eye on the right. Top panel shows enlarged blind spots only, which reflects the earliest VF findings in IIH patients. The bottom panel shows a more advanced presentation with characteristic arcuate pattern of loss with eventual central involvement as seen here in the left eye. Patients progress over days to weeks in some cases, and over several months in others if undertreated. Predominant central or centrocecal VF loss without arcuate changes should raise suspicion for alternative diagnoses such as inflammatory, infectious, infiltrative, metabolic/toxic/nutritional optic neuropathies.
Testing
Patients suspected of having IIH on history require a complete ophthalmological examination including formal visual fields and dilated fundus examination. Fundus photography and OCT RNFL can also be very helpful in following these patients.
All patients in whom papilledema is suspected on clinical examination, should have a blood pressure taken in the office. Hypertensive papillopathy can present identically to IIH, without associated retinal or choroidal hypertensive findings.
All patients with suspected papilledema must have a neuroimaging study done prior to lumbar puncture. The study is done not only to ensure the absence of a space-occupying lesion that could cause brain herniation upon lumbar puncture; it also can identify hydrocephalus, hemorrhage, and other anomalies that change the diagnostic workup. Magnetic resonance imaging (MRI) with contrast enhanced MR venography (CE-MRV) is the most complete study to obtain in patients with suspected IIH (see below). However, in the acute setting, it might be difficult to get an MRI that will be done with the specific sequences and quality desired. Thus, a computed tomography (CT) scan of the head should be performed on the day of the diagnosis of bilateral optic disc swelling if there is any delay in obtaining an MRI/MRV.
Several recent studies have looked more closely at the issue of whether certain findings on MRI can be reliably correlated with IIH; essentially, ruling in the diagnosis of IIH instead of ruling out other causes of papilledema. These findings are not suggested to be specific for IIH, but might be present in the absence of features suggestive of an alternate etiology for raised ICP, such as enlarged ventricles in hydrocephalus, pachymeningeal thickening in meningitis, or signs of dural venous thrombosis. Listed below are the various features that have been described in the literature.
MRI findings potentially suggestive of raised intracranial pressure
- Flattening of the posterior aspect of the globe(s)
- Dilated perioptic fluid cuff
- Vertical or horizontal tortuosity of the optic nerves
- Partially empty sella
- Posterior displacement of the pituitary stalk
- Flattened course of the optic chiasm
- Tight subarachnoid spaces
- Slit-like ventricles
- Inferior displacement of the cerebellar tonsils
- Optic nerve protrusion
- Significant venous stenosis
In a recent MRI/MRV study analyzing patients with known IIH, we found that flattening of the posterior aspect of the globe, partially empty sella, and significant venous stenoses to be most sensitive and specific signs, when taken within the appropriate clinical context. We have found looking for these features helpful in cases in which the typical clinical signs and symptoms of IIH are absent or ambiguous.
Lumbar puncture is mandatory for the diagnosis of PTC, not only to confirm that the opening pressure is elevated, but also to ensure that the cerebrospinal fluid formula is normal. A number of inflammatory or infectious conditions can present with disc swelling and signs and symptoms of elevated intracranial pressure, and diagnosis of these conditions when they exist is imperative. Some patients will have a normal opening pressure at the time of LP but have signs/symptoms of elevated intracranial pressure. Such patients might benefit from intracranial pressure monitoring (inpatient study, done for 24–48 hours). As with blood pressure and IOP, intracranial pressure can vary diurnally.
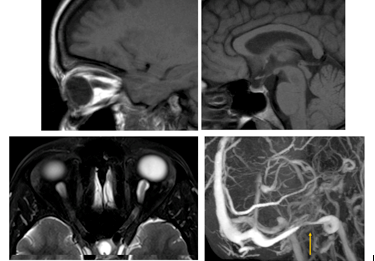
Figure 4. MRI findings often found in patients with raised intracranial pressure include vertical tortuosity of the optic nerve and flattening of the posterior aspect of the globe as seen in the top left image. Top right shows the appearance of a partially empty sella, while the bottom left image is an example of dilation of the perioptic fluid cuff. A typical distal transverse sinus stenosis at its junction with the sigmoid sinus is demonstrated in the bottom right frame.
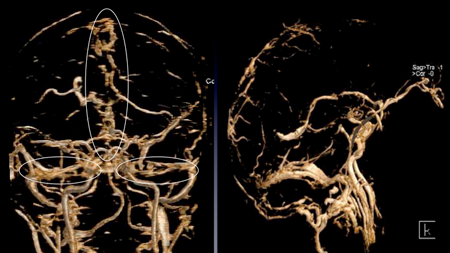
Figure 5. Cerebral venous sinus thrombosis (CVST). In contrast to the venous sinus stenoses seen in IIH, thromboses typically demonstrate irregular, patchy filling and lack a specific predilection for involvement of the transverse/sigmoid sinus junction. Note the irregularities within the superior sagittal and transverse sinuses (left image) and lack of filling of the straight sinus (right image).
Testing for staging, fundamental impairment
As mentioned above, the stage of papilledema might not correlate in some cases with the degree of visual impairment. Hence, functional testing including visual acuity, color vision and most importantly, formal perimetry are essential for accurate assessment in IIH patients. Some patients might have a degree of optic atrophy and gliosis (overlying scarring) from chronic, longstanding papilledema. In the face of persistent or recurrent ICP elevation, such nerves might not demonstrate the classical features of papilledema seen in “fresh” cases. Hence, attention to retinal venous tortuosity, shunt vessels, and neuroimaging findings (discussed above) might be fruitful.
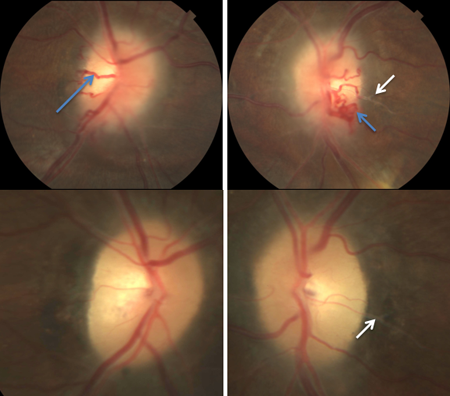
Figure 6. Chronic papilledema. Top panel shows a patient with active yet chronic elevation of ICP with optociliary shunt vessels (blue arrows) and RNFL swelling, peripapillary fluid and gliosis extending overlying the disc and retinal vessel just temporal to the disc (white arrows). The bottom panel shows fundus photos of the same patient following a ventriculoperitoneal shunt placement. Note disappearance of the shunt vessels, underlying optic atrophy and persistence of gliosis.
OCT RNFL and GCC (ganglion cell complex) can be very helpful in following IIH patients, with the exception of cases with RNFL thickening above 250–300 microns, where the utility of OCT diminishes. OCT is a helpful adjunct when correlating the degree of RNFL thickening with VF involvement. For example, if OCT demonstrates a reduction in RNFL thickness in a patient with improving visual fields, this suggests that edema is improving along with visual function as expected. However, in cases of reduction of RNFL thickness on OCT and accompanying deterioration of VF, the more likely conclusion is loss of RNFL volume due to atrophy. GCC analysis is especially good at resolving the integrity of the underlying ganglion cell layer, which is not affected by overlying edema; hence, GCC assessment provides a more accurate measure of retinal architecture and better correlation with visual function.
Risk factors
In addition to obesity and gender, as mentioned earlier, a number of risk factors exist. Secondary causes of raised intracranial pressure include
- Physically obstructive lesions or conditions
- Obstructive hydrocephalus
- Chiari I malformation)
- Numerous drugs including
- Tetracycline
- Minocycline
- Doxycycline
- Hormone: human growth hormone, thyroxine, leuprorelin acetate, levonorgestrel, anabolic steroids
- Withdrawal from chronic corticosteroid therapy
- Vitamin A and related retinoids
- Topical formulations
- Accutane
- ATRA (all-trans retinoic acid, used in promyelocytic leukemia)
- Impaired venous drainage due to venous sinus thrombosis or stenosis
A potential association with oral contraceptive formulations remains controversial. It is especially important to consider these secondary causes of intracranial hypertension in patients who do not fit the standard demographics; this population includes all men (regardless of weight), nonobese women, and women over the age of 45 (again, regardless of weight/obesity).
To some extent, the workup described above applies to all patients, and the distinction in the initial workup is not too important; in other words, all patients get the same initial history and examination, followed by appropriate neuroimaging studies to look for underlying secondary causes of this syndrome. The purpose of mentioning the “nontraditional” PTC patients is to re-emphasize the need in all patients to look for a cause that might be treated specifically rather than passively through ICP lowering alone. For example cessation of an offending medications such as a vitamin A derivative or minocycline can reverse the condition entirely without need for additional treatment. Obstructive sleep apnea also seems to be associated with IIH, presumably through the mechanism of increased CO2 trapping and subsequent cerebral and venous congestion.
Differential diagnosis
IIH remains a diagnosis of exclusion in patients suspected of having papilledema. Important diagnostic considerations include intra- or extra-axial space-occupying lesions, hydrocephalus, meningitis, intracranial hemorrhage, and venous sinus thrombosis.
As mentioned earlier, the possibility of malignant hypertension must always be considered in cases of bilateral disc swelling with relatively normal visual function, at least acutely. Appropriate imaging studies and subsequent lumbar puncture if indicated should allow one to differentiate these entities from IIH and each other.
All of the above entities should generally present with minimal afferent visual dysfunction, with the exception of blind-spot enlargement or arcuate visual field loss. Bilateral disc swelling encountered in association with significant VA decrease, color vision deficit, or central involvement in VF suggests potentially inflammatory, infectious, or infiltrative conditions.
Pseudopapilledema is a common entity to consider in the differential diagnosis of papilledema. The diagnostic uncertainly often arises in the context of buried optic nerve head drusen, especially in children where the drusen have not yet calcified, making orbital CT scans and ultrasound less helpful. ONHD patients should not reveal any RNFL hemorrhages or obscuration of retinal vessels. OCT can be helpful as well, given increasing ability to resolve these deposits directly using enhanced depth imaging (EDI OCT) and a generally thinner nasal RNFL (< 80 microns) than typically seen in papilledema.
Patient management: treatment and follow-up
The management of IIH can be straight-forward in some cases and quite challenging in others. The following discussion lays out the options and important considerations in decision-making.
Natural history
Because most cases of IIH are still in the idiopathic category, treatment is directed at lowering the intracranial pressure. Some cases of IIH are managed effectively with weight loss alone, supporting the role of this very powerful association. Studies have shown that a relatively modest degree of weight loss (6% or less) alone or reduced calorie diets (< 1250 calories/day for 6 weeks) can have a dramatic effect on papilledema, visual fields and ICP symptoms, without adjuvant treatment. Hence weight loss remains the over-arching consideration, mainstay of therapy and compliments all other forms of treatment as well.
Once the diagnosis is made, and therapy is instituted patients are typically followed every 10–12 weeks, with complete dilated examination, automated visual fields, and OCT RNFL at each visit. If there is cause for concern based on severity of clinical findings at presentation, or subjective deterioration, it is best to follow these patients more closely.
Medical therapy
Short-term medical management is most commonly initiated with acetazolamide (Diamox), which must be prescribed at the proper frequency and dose to have any real effect on intracranial pressure. Even in severe disc edema cases with dramatic elevation of ICP, medical management is often tried for a week or two as some patients are able to avoid surgical intervention with aggressive (2–3 grams of Diamox daily) medical management. Patients should be monitored very closely during this initial period. However, if advanced visual loss is present at presentation (constricted VFs, visual acuity loss attributable to optic neuropathy and not macular edema) a surgical approach should be considered at the outset.
At a minimum, adult patients of normal body weight or greater (the vast majority of patients) will require a daily dose of at least 1000 mg, although some patients respond to less. Acetazolamide has a short half-life, and if given in standard tablet form, should be dosed 4 times a day. Diamox sequels can be given twice a day. Sulfa allergy is a frequent consideration when prescribing Diamox. While acetazolamide also is a sulfonamide derivative, its chemical structure lacks the antigenic moiety associated with allergy. Reports of true allergy to acetazolamide in patients with a previously documented sulfonamide antibiotic allergy are rare to nonexistent in the literature. Patients intolerant of Diamox due to malaise, depressed mood, metallic taste, parasthesias, potassium depletion, renal insufficiency, or rarely bone marrow depression, can try a weaker CA inhibitor, neptazane. However, this is likely a less effective medication than Diamox. A randomized controlled clinical trial recently demonstrated the increased effectiveness of Diamox for the treatment of IIH patients with mild to moderated visual field loss, compared to weight loss alone (JAMA. 2014;311(16):1641-1651)
Some patients are prescribed Topamax (topiramate) as a first line agent or eventually within their course of therapy for IIH. Topiramate is also a sulfonamide derivative whose primary indications are antiepileptigenesis and migraine prophylaxis. Its efficacy in IIH probably derives from its beneficial effects on headache (about 2/3rds of IIH patients have migraine or tension type headaches that are not pressure-dependent) and body weight (weight loss occurs in many patients from decreased appetite and increased metabolism) rather than its weak carbonic anhydrase inhibitory activity. A common side effect of topiramate is cognitive slowing, especially at higher doses (> 150 mg/day). This medication can be very helpful for patients who in the course of their condition (once ICP has normalized) begin to suffer from headache that is different in character from their initial ICP elevation type headache. Simply increasing Diamox dosage in such patients with persistent headache should be avoided unless the headache is similar in quality to initial presentation and ICP is suspected to be elevated based on clinical findings.
Surgery
Surgical management remains second-line treatment in patients who fail medical therapy or weight loss. There are no randomized trials or even adequately powered prospective studies comparing outcomes of surgical interventions in IIH to guide our management in a truly evidenced-based manner. Usually it is the patients with acute loss of vision or intractable headache who become surgical candidates. Both optic nerve sheath fenestration (ONSF) and ventriculo-peritoneal (VP) shunting should be considered based on the clinical picture. Although lumbo-peritoneal shunting can be helpful as well, VPS is generally favored given the increased tendency for failure of LP shunts and possibility of worsening foramen magnum crowding due to downward herniation of the cerebellar tonsils.
Many consider ONSF the procedure of choice where vision loss is the primary concern in cases of active papilledema. Others have reported headache relief after ONSF, but the mechanism by which this might occur is unclear. Unilateral ONSF can lead to bilateral improvement in papilledema, but this effect is variable, and bilateral sequential surgery is often necessary. Papilledema should start to resolve within days, and complete resolution can be seen in 4–6 weeks. The main measure of success (or lack of it) is improvement in VF or cessation of further deterioration. When headache is a more prominent (or the primary) issue, then VP shunting should be favored. A shunt has the advantage of potentially treating both headache and papilledema; headache is more likely to respond to shunting if it is not longstanding (> 2 years).
Both ONSF and VP shunting tend to fail with time; persistent CSF drainage in ONSF probably lasts 6 months or less. Concerns about direct optic nerve toxicity have limited the use of mitomycin C or other agents to prevent scarring. It is possible that the scarring itself is beneficial, as the optic nerve head would then become insulated from the CSF compartment pressure. Because ONSF is used to prevent acute vision loss, its long-term persistence might be less important. VP shunts also tend to fail with time, as almost 50% of patients will require one or more shunt revisions within 5 years. Whenever an IIH patient with a shunt experiences recurrent symptoms, shunt failure or infection must be considered and shunt patency studies ordered.
Other management considerations
Venous sinus stenting shows promise in the treatment of secondary IIH from venous outflow obstruction, with worldwide evidence of therapeutic efficacy. Stenting is most successful where there is focal narrowing of the venous channel without extrinsic compression. Demonstration of a pressure gradient across the stenosis should precede the stenting procedure, as this measurement helps to confirm that the stenosis is in fact hemodynamically significant. The stent is deployed and the gradient remeasured. Patients must remain on antiplatelet therapy for at least 6 months after the procedure; pregnancy likely should be avoided during this time because of the drugs being used (aspirin and/or clopidogrel). Although complications are quite rare (2 of 52 patients experienced an adverse event in the largest series to date; Ahmed et al AJNR Am J Neuroradiol. 2011;32:1408–1414), important considerations include subarachnoid hemorrhage, venous infarct, or restenosis or thrombosis at the edges of the stent.
Many centers throughout the world are working on protocols to guide the decision-making process when considering various surgical interventions in complex IIH patients.
IIH in pregnancy can present a number of challenges and special management considerations. Although patients will necessarily gain weight and experience physiological and hormonal changes, most patients do not require urgent interventions for pre-existing IIH or IIH that develops during pregnancy. Careful monitoring every 4–6 weeks with examination and VF testing is advised. Diamox is considered safe to use after 20 weeks of gestation; however, it remains a class-C medication. Most patients do not require treatment. In cases with progressive VF loss and worsening papilledema, serial (weekly LPs can be done although this can be difficult from a practical point of view. ONSF or less preferably VPS (given theoretical peritoneal catheter complications) are needed in rare circumstances of advancing visual loss. Corticosteroids can also be used in pulse doses as needed. No special provisions are required in terms of delivery, although most high risk obstetricians prefer Caeserian section in such cases. Close follow up and communication with the patient’s obstetrician is imperative.
In morbidly obese patients (BMI > 40 or BMI > 35 with a comorbid disease), bariatric surgery should be considered seriously if conservative measures do not give adequate results. Many patients with IIH who undergo bariatric surgery have shown a dramatic improvement or resolution of their IIH findings. Referral to a weight management clinic for consideration of all options can be very helpful.
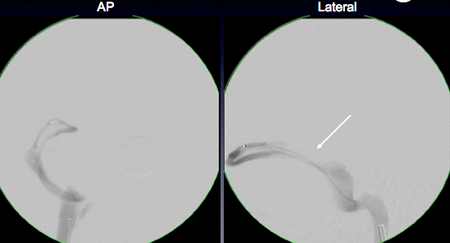
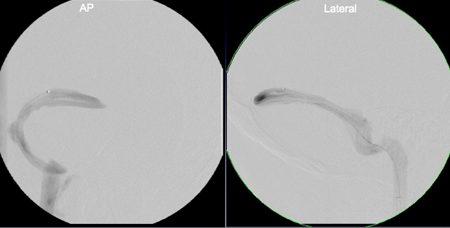
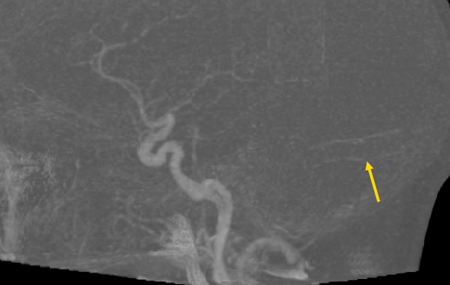
Figure 7. Retrograde venous manometry of the cerebral venous sinuses in a patient with IIH and observed transverse sinus stenosis on MRV. Top panel shows the area of focal pressure gradient from 25 mm Hg to 14 mg Hg (arrow) across the stenosis. This region was stented, with subsequent reduction of the gradient 2 mm (17 mg Hg to 15 mm Hg) across the stent (middle panel). The bottom panel shows plain radiograph of the stent (arrow).
Disease-related complications
Some patients unfortunately present with advanced visual field loss, optic atrophy and very little salvageable vision. All attempts should be made in even advanced cases to normalize ICP and optic nerve compromise as much as possible to prevent further visual loss. Patients can be left NLP with this condition in severe cases.
Early diagnosis, addressing all potentially impactful risk factors (medications, weight, OSA), and deliberate institution of therapy should limit the degree of morbidity associated with this condition. Interventions should be as aggressive as necessary with close follow up throughout to limit permanent neurological and visual sequelae.
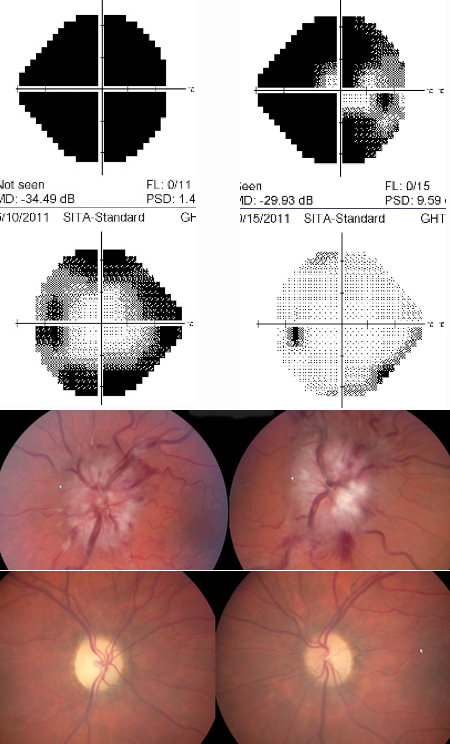
Figure 8. Left visual field and top fundus show a patient with severe papilledema and advanced visual field loss OU, greater involvement OD than OS. The patient underwent emergent bilateral optic nerve sheath fenestrations; however, the right VF and bottom fundus reveal optic atrophy OU and residual VF loss, although improved from preoperative function. Courtesy Prem Subramanian.
References and additional resources
- Walsh and Hoyt’s Clinical Neuro-ophthalmology: The Essentials, 3rd edition, LWW 2015. Editors: Miller NR, Subramanian PS, Patel VR.
- nanosweb.org. Patient information on IIH written and endorsed by the North American Neuro-ophthalmology Society.
- Abel AS, Brace JR, McKinney AM, Harrison AR, Lee MS. Practice Patterns and Opening Pressure Measurements Using Fluoroscopically Guided Lumbar Puncture. American Journal of Neuroradiology. 2012.
- Abtahi M-A, Abtahi S-H, Fazel F, et al. Topiramate and the vision: a systematic review. Clin Ophthalmol. 2012;6:117–131.
- Ahmed R, Wilkinson M, Parker G, Thurtell M, Macdonald J, McCluskey P, Allan R, Dunne V, Hanlon M, Owler B, Halmagyi GM. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol. 2011;32:1408–1414
- Alsuhaibani AH, Carter KD, Nerad JA, Lee AG. Effect of Optic Nerve Sheath Fenestration on Papilledema of the Operated and the Contralateral Nonoperated Eyes in Idiopathic Intracranial Hypertension. Ophthalmology. 2011;118(2):412–414.
- Arac A, Lee M, Steinberg GK, Marcellus M, Marks MP. Efficacy of endovascular stenting in dural venous sinus stenosis for the treatment of idiopathic intracranial hypertension. Neurosurg Focus. 2009;27(5):E14.
- Arber N, Shirin H, Fadila R, Melamed E, Pinkhas J, Sidi Y. Pseudotumor cerebri associated with leuprorelin acetate. Lancet. 1990;335(8690):668.
- Avery RA, Mistry RD, Shah SS, et al. Patient position during lumbar puncture has no meaningful effect on cerebrospinal fluid opening pressure in children. J Child Neurol. 2010;25(5):616–619.
- Avery RA, Shah SS, Licht DJ, et al. Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med. 2010;363(9):891–893.
- Bababeygy SR, Repka MX, Subramanian PS. Minocycline-associated pseudotumor cerebri with severe papilledema. J Ophthalmol. 2009;2009:203583.
- Banik R, Lin D, Miller NR. Prevalence of Chiari I malformation and cerebellar ectopia in patients with pseudotumor cerebri. J Neurol Sci. 2006;247(1):71–75.
- Bono F, Lupo MR, Serra P, et al. Obesity does not induce abnormal CSF pressure in subjects with normal cerebral MR venography. Neurology. 2002;59(10):1641–1643.
- Bussiere M, Falero R, Nicolle D, Proulx A, Patel V, Pelz D. Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2010;31:645–650
- Chewning RH, Murphy KJ. Gadolinium-based contrast media and the development of nephrogenic systemic fibrosis in patients with renal insufficiency. J Vasc Interv Radiol. 2007;18(3):331–333.
- Corbett JJ, Mehta MP. Cerebrospinal fluid pressure in normal obese subjects and patients with pseudotumor cerebri. Neurology. 1983;33(10):1386–1388.
- Digre KB, Nakamoto BK, Warner JEA, Langeberg WJ, Baggaley SK, Katz BJ. A comparison of idiopathic intracranial hypertension with and without papilledema. Headache. 2009;49(2):185–193.
- Donnelly SJ, Subramanian PS. Relationship of intraocular pulse pressure and spontaneous venous pulsations. Am J Ophthalmol. 2009;147(1):51–55.e2.
- Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol. 1988;45(8):875–877.
- Farb RI, Scott JN, Willinsky RA, Montanera WJ, Wright GA, terBrugge KG. Intracranial venous system: gadolinium-enhanced three-dimensional MR venography with auto-triggered elliptic centric-ordered sequence–initial experience. Radiology. 2003;226(1):203–209.
- Feldon SE. Visual outcomes comparing surgical techniques for management of severe idiopathic intracranial hypertension. Neurosurg Focus. 2007;23(5):E6.
- Fera F, Bono F, Messina D, et al. Comparison of different MR venography techniques for detecting transverse sinus stenosis in idiopathic intracranial hypertension. J Neurol. 2005;252(9):1021–1025.
- Fridley J, Foroozan R, Sherman V, Brandt ML, Yoshor D. Bariatric surgery for the treatment of idiopathic intracranial hypertension. J Neurosurg. 2011;114(1):34–39.
- Friedman DI1, Liu GT, Digre KB Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children.Neurology. 2013 Sep 24;81(13):1159-65
- Horton JC, Seiff SR, Pitts LH, Weinstein PR, Rosenblum ML, Hoyt WF. Decompression of the optic nerve sheath for vision-threatening papilledema caused by dural sinus occlusion. Neurosurgery. 1992;31(2):203–11; discussion 211–2.
- Jacks AS, Miller NR. Spontaneous retinal venous pulsation: aetiology and significance. J Neurol Neurosurg Psychiatr. 2003;74(1):7–9.
- Johnson LN, Diehl ML, Hamm CW, Sommerville DN, Petroski GF. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009;127(1):45–49.
- Johnson LN, Krohel GB, Madsen RW, March GA. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri). Ophthalmology. 1998;105(12):2313–2317.
- Johnston I, Kollar C, Dunkley S, Assaad N, Parker G. Cranial venous outflow obstruction in the pseudotumour syndrome: incidence, nature and relevance. J Clin Neurosci. 2002;9(3):273–278.
- Karam EZ, Hedges TR. Optical coherence tomography of the retinal nerve fibre layer in mild papilloedema and pseudopapilloedema. Br J Ophthalmol. 2005;89(3):294–298.
- Kesler A, Goldhammer Y, Hadayer A, Pianka P. The outcome of pseudotumor cerebri induced by tetracycline therapy. Acta Neurol Scand. 2004;110(6):408–411.
- Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50(4):1094–1098.
- Kurz-Levin MM, Landau K. A comparison of imaging techniques for diagnosing drusen of the optic nerve head. Arch Ophthalmol. 1999;117(8):1045–1049.
- Lascaratos G, Ahmed S, Madill SA. Pearls & Oy-sters: spontaneous venous pulsation and its role in differentiating papilledema from pseudopapilledema. Neurology. 2010;75(13):e53–4.
- Leach JL, Fortuna RB, Jones BV, Gaskill-Shipley MF. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics. 2006;26 Suppl 1:S19–41; discussion S42–3.
- Lee AG, Anderson R, Kardon RH, Wall M. Presumed “sulfa allergy” in patients with intracranial hypertension treated with acetazolamide or furosemide: cross-reactivity, myth or reality? Am J Ophthalmol. 2004;138(1):114–118.
- Loring DW, Williamson DJ, Meador KJ, Wiegand F, Hulihan J. Topiramate dose effects on cognition: A randomized double-blind study. Neurology. 2010.
- Maralani PJ, Hassanlou M, Torres C, Chakraborty S, Kingstone M, Patel V, Zackon D, Bussière M. Accuracy of brain imaging in the diagnosis of idiopathic intracranial hypertension. Clin Radiol. 2012 Jul;67(7):656-63.
- McGirt MJ, Woodworth G, Thomas G, Miller N, Williams M, Rigamonti D. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: predictors of treatment response and an analysis of long-term outcomes. J Neurosurg. 2004;101(4):627–632.
- Meckel S, Reisinger C, Bremerich J, et al. Cerebral Venous Thrombosis: Diagnostic Accuracy of Combined, Dynamic and Static, Contrast-Enhanced 4D MR Venography. AJNR American journal of neuroradiology. 2009.
- Nedelmann M, Kaps M, Mueller-Forell W. Venous obstruction and jugular valve insufficiency in idiopathic intracranial hypertension. J Neurol. 2009;256(6):964–969.
- Owler BK, Parker G, Halmagyi GM, et al. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg. 2003;98(5):1045–1055.
- Paquet C, Poupardin M, Boissonnot M, Neau JP, Drouineau J. Efficacy of unilateral stenting in idiopathic intracranial hypertension with bilateral venous sinus stenosis: a case report. Eur Neurol. 2008;60(1):47–48.
- Purvin V, Kawasaki A. Neuro-ophthalmic emergencies for the neurologist. Neurologist. 2005;11(4):195–233.
- Rebolleda G, Muñoz-Negrete FJ. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50(11):5197–5200.
- Richmond H, Zwerner J, Kim Y, Fiorentino D. Nephrogenic systemic fibrosis: relationship to gadolinium and response to photopheresis. Arch Dermatol. 2007;143(8):1025–1030.
- Shaia JK, Sharma N, Kumar M, Chu J, Maatouk C, Talcott K, Singh R, Cohen DA. Changes in Prevalence of Idiopathic Intracranial Hypertension in the United States Between 2015 and 2022, Stratified by Sex, Race, and Ethnicity. Neurology. 2024 Feb 13;102(3):e208036. doi: 10.1212/WNL.0000000000208036. Epub 2024 Jan 5. PMID: 38181397; PMCID: PMC11097766.
- Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701.
- Skau M, Milea D, Sander B, Wegener M, Jensen R. OCT for optic disc evaluation in idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol. 2010.
- Spoor TC, McHenry JG, Shin DH. Long-term results using adjunctive mitomycin C in optic nerve sheath decompression for pseudotumor cerebri. Ophthalmology. 1995;102(12):2024–2028.
- Spoor TC, McHenry JG. Long-term effectiveness of optic nerve sheath decompression for pseudotumor cerebri. Arch Ophthalmol. 1993;111(5):632–635.
- Streams BN, Liu V, Liégeois N, Moschella SM. Clinical and pathologic features of nephrogenic fibrosing dermopathy: a report of two cases. J. Am. Acad. Dermatol. 2003;48(1):42–
- Subramanian PS, Goldenberg-Cohen N, Shukla S, Cheskin LJ, Miller NR. Plasma ghrelin levels are normal in obese patients with idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol. 2004;138(1):109–113.
- Sugerman HJ, Felton WL III, Sismanis A, et al. Continuous negative abdominal pressure device to treat pseudotumor cerebri. Int. J. Obes. Relat. Metab. Disord. 2001;25(4):486–490.
- Sugerman HJ, Felton WL, Sismanis A, Kellum JM, DeMaria EJ, Sugerman EL. Gastric surgery for pseudotumor cerebri associated with severe obesity. Ann. Surg. 1999;229(5):634–40; discussion 640–2.
- Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593–617.
- Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri). Curr Neurol Neurosci Rep. 2008;8(2):87–93.
- Wang MTM, Bhatti MT, Danesh-Meyer HV. Idiopathic intracranial hypertension: Pathophysiology, diagnosis and management. J Clin Neurosci. 2022 Jan;95:172-179. doi: 10.1016/j.jocn.2021.11.029. Epub 2021 Dec 17. PMID: 34929642.
- Warner JEA, Larson AJ, Bhosale P, et al. Retinol-binding protein and retinol analysis in cerebrospinal fluid and serum of patients with and without idiopathic intracranial hypertension. Journal of Neuro-Ophthalmology. 2007;27(4):258–262.
- Yiğit H, Turan A, Ergün E, Koşar P, Koşar U. Time-resolved MR angiography of the intracranial venous system: an alternative MR venography technique. Eur Radiol. 2011
- Bussiere M, Falero R, Nicolle D, Proulx A, Patel V, Pelz D. Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2010;31:645–65
