Lentigo Maligna, Lentigo Maligna Melanoma, and Melanocytic Hyperplasia
Updated August 2024
For the clinician encountering acquired melanocytic skin lesions, the potential for existence or development of invasive melanoma must always be considered. The spectrum of melanocytic lesions ranges from senile lentigo with no atypia to melanocytic hyperplasia and mild-severe atypia to lentigo maligna, which features malignant cells confined to the epidermis. Finally Lentigo maligna melanoma is a presentation in which the epidermis has been breached by malignant cells. This outline reviews our understanding of the biologic behavior and current treatment considerations for this spectrum of acquired disease. Newer technology, such as in vivo reflectance confocal microscopy, is also reviewed.
Establishing the diagnosis
Etiology
Possibly actinic induced hyperplasia of hyperpigmented and pleomorphic melanocytes
Epidemiology
- Age greater than 50 (peak incidence in seventh to eighth decades)
- Primarily confined to sun-exposed skin: head and neck region, and hands
- Living in areas with high ultraviolet (UV) exposure
- Fair skin, often with history of severe sunburns
- Occupational risk with increased hours of sun exposure
- In the US, the incidence of lentigo maligna is greatest in Hawaii, intermediate in the central and southern states, and lowest in the northern states
- In a series from Stanford, lentigo maligna and lentigo maligna melanoma (see below) were the only melanoma subtypes increasing in incidence from 1990 to 2000. (Swetter et al., J Invest Dermatol 2005)
History
- Previous history of actinic pre-cancerous or malignant skin lesions
- Pigmented skin lesion in sun-exposed area (face, forearms) that has slowly increased in size or changed in color
Clinical features
- The term “lentigo maligna” (LM) is still used today by pathologists to refer to a melanoma in situ that occurs in severely sun-damaged skin. “Lentigo maligna melanoma” (LMM) refers to a melanoma-in-situ that has minimally escaped from the epidermis. Many studies group the two entities together. Lentigo maligna melanoma (most often found in the head and neck) is 1 of the 4 main subtypes of invasive melanoma and represents 5%–30% of all melanoma cases
- As is true for all melanomas in situ on all anatomic sites, the lentigo maligna lesions tend to be
- Flat or very slightly elevated
- Asymmetrical, irregularly shaped
- Notched in outline
- Highly characteristic uneven pigmentation with colors that range from light brown to black (Figure 1)
- Macular pigmented skin lesion (flat, not elevated nodule)
- Asymmetric lesion with irregular borders
- Significant variation in pigment (light brown to black)
- Frequently located in malar area
- Progression to invasive melanoma
- If untreated, lentigo maligna may invade into the dermis or subcutaneous fat, progressing to vertically invasive lentigo maligna melanoma (LMM). The true risk of progression from LM to LMM is unclear; it has been estimated to be as high as 30% to 50% or as low as 5% from epidemiologic studies (Cohen 1995).
- Weinstock and Sober (Br J Dermatol 1987) used incidence and prevalence metadata to estimate lifetime risk of progression from LM to LMM to be 4.7% for a 45-year-old; however, some of the data was based on clinical and not pathologic diagnoses.
- Malhotra et al. (Ophthalmology 2003) reported that 75% of the LMM found in their series were clinically thought to be LM only, again indicating a possibly higher progression rate.
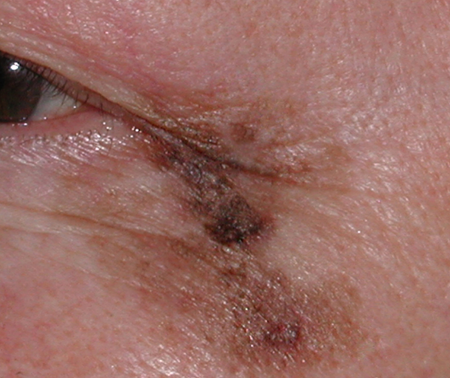
Figure 1. Clinical photo of lentigo maligna lesion with irregular borders and variable pigmentation.
Testing
- Pathological examination of the entire excised specimen
- Biopsy should include full-thickness skin extending to the subcutaneous fat (punch biopsy). Superficial skin biopsy by shaving, scissors, or curettage, does not allow for assessment of tumor thickness, which is important for prognostication and treatment planning.
- Excisional biopsy with a narrow margin of normal-appearing skin is optimal, which can usually be performed. Incisional biopsy is indicated for large lesions that cannot be completely excised without significant deformity.
- Lentigo maligna consists of a proliferation of atypical melanocytes in the basal layer of the epidermis and within the hair follicle, hence considered “in situ” as it is confined to the tissue of origin.
- As the tumor progresses, melanocyte density and degree of cytological atypia increase
- Advanced cases of LM often demonstrate confluent melanocyte growth along the basal epidermis, with nest formation, pagetoid epidermal invasion, and florid adnexal involvement
- Signs of sun damage, i.e., marked solar elastosis and epidermal atrophy, are often present in the upper dermis
- Negative histologic margins are the standard of care in the treatment of LM and LMM.
- Despite the increasing understanding of melanocytic lesions, the accuracy of frozen section histology in assessing melanoma excision margins during Mohs micrographic surgery continues to be highly debatable. Therefore, immunohistochemical (IHC) staining for melanocyte markers has become an important element in ensuring accuracy of margin evaluation, particularly for the removal of lentigo maligna (LM) and early stage lentigo maligna melanoma (LMM), subtypes of melanoma known for their indistinct clinical borders and asymmetrical subclinical extensions.
- Stains used include hematoxylin and eosin, and immunohistochemical stains summarized below. Two markers that are emerging to be more promising are melanoma antigen recognized by T cells (MART-1), also known as Melan-A antigen (Melan-A), and microphthalmia-associated transcription factor (MiTF). (Figures 2 and 3)
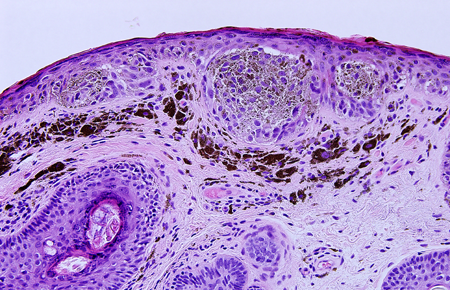
Figure 2. Histopathologic specimen demonstrating classic lentigo maligna/melanoma in situ on H & E stain (20X). An increased number of melanocytes at the dermal-epidermal junction, with some forming nests, as well as mildly enlarged, hyperchromatic nuclei are seen.
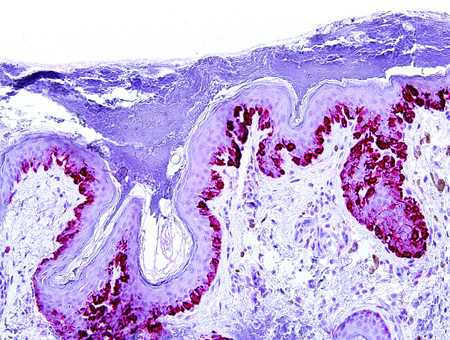
Figure 3. Example of a positive margin on the right specimen edge following Slow Mohs excision. Melan-A/MART-1 immunohistochemical staining strongly highlights an increased number of melanocytes at the dermal-epidermal junction, with multifocal confluence and scattered structures, consistent with small nests of abnormal cells. There is also prominent extension along adnexal epithelium.
- Immunohistochemical stains for melanocytes:
- MART-1 (Melan-A): is a melanocyte differentiation antigen, a cytoplasmic melanosome-associated glycoprotein, expressed in 80% to 100% of melanomas, resting adult melanocytes, and nevus cells in epidermal and dermal compartments (Busam, Am J Surg Pathol 1998). This stain highlights every melanocyte so is an excellent means of assessing pagetoid spread, margins and extent of the lesion.
- Some studies have shown that MART-1/Melan-A may overestimate the number of melanocytes present due to non-specific staining of pigmented keratinocytes in the setting of inflamed lichenoid areas, resulting in a false positive margin and an overestimation of intraepidermal melanocytes (El Shabrawi-Caelen, Am J Dermatopathol 2004; Beltraminelli, Am J Dermatopathol 2009; Kim, J Cutan Pathol 2011).
- The uncommon variant, spindle cell melanoma or desmoplastic melanoma, does not stain positive with MART-1/Melan-A.
- Human melanoma black (HMB-45): HMB-45 is often diffusely positive in melanoma; zonal staining, in which there is diminished staining in deeper parts of the lesion, is more suggestive, but in no way diagnostic, of a nevus.
- Stains normal melanocytes poorly and can show patchy staining of atypical intraepidermal melanocytes, resulting in decreased sensitivity
- S-100 protein: Very sensitive. S-100 is useful if you are not sure if the tumor is melanocytic or another cell type
- Sry-related HMG-BOX gene 10 (SOX10): a nuclear stain for melanocytes and schwann cells. It is less likely to be expressed by background fibrocytes and histiocytes than S-100. Excellent choice if you are not sure if the tumor is melanocytic or another cell type.
- Microphthalmia transcription factor (MiTF) has both high sensitivity and specificity for melanocytes, particularly intraepidermal melanocytes. MiTF is a transcription factor that is important in the development of neural crest-derived melanocytes, and in melanocyte survival.
- Some studies have shown MiTF immunohistochemical staining to have high sensitivity (81%–100%) and specificity (88%–100%) for melanoma, but may also stain various dermal inflammatory cells, histiocytes, and fibrocytes as well (King, Am J Surg Pathol 2001)
- MiTF has recently been shown to be as effective as MART-1 in recognizing melanocytes in melanoma in situ (Hillesheim 2011). In addition, nuclear MiTF labeling may better approximate the total number of melanocytes present, as compared to visual counts, especially in subtle lentiginous intraepidermal melanocytic lesions (Kim, J Cutan Pathol 2011)
- Ki-67 (MIB-1): this may be useful in distinguishing benign melanocytic nevi (where less than 5% of melanocytes are positive) from melanoma (more than 25% are positive). These percentages are rough guidelines and by no measure absolutely diagnostic. Because assessing the percentage is so subjective and counting positive cells is so tedious, computer software has evolved to aid in this assessment
- Mel-5 identifies both normal and abnormal intraepidermal melanocytes, but is less reliable in identifying dermal melanocytes
- Even among experienced dermatopathologists, it can be difficult to reproducibly grade atypia among melanocytes. Areas of lentigo maligna may border areas of not-quite-malignant melanocytic hyperplasia with variable atypia, which may border additional areas of lentigo maligna in severely sun-damaged individuals. Clinical management can therefore be complex, especially since the exact risk of progression to invasive melanoma is unknown for melanocytic hyperplasia with atypia.
Testing for staging, fundamental impairment
- Prognosis for invasive lentigo maligna melanoma does not differ significantly from that of other histogenetic types of melanoma after controlling for tumor thickness
- If invasive melanoma and not lentigo maligna, then metastatic evaluation includes liver function tests, serum lactose dehydrogenase, full-body imaging (PET, CT, MRI)
- For invasive melanoma, consider sentinel lymph node biopsy, to assess regional lymph node involvement and to decide on adjuvant therapy. This is indicated in all melanoma patients except those with stage 0 or stage 1A disease (patients with a lesion less than 1 mm)
Risk factors
- Sun exposure
- Fair skin with history of severe sunburns
- Occupational risks
- Increased number of melanocytic nevi, including large or giant congenital nevi
- Family history of malignant melanoma
- Gene on chromosome arm 9p encodes a tumor suppressor gene called CDKN2A or MTS1; another gene identified in melanoma-prone families is CDK4
Differential diagnosis
- Malignant melanoma
- Benign freckle (ephelis)- very small, flat, uniformly brown spots with sharp borders that represent hyperpigmentation of the basal layer of the epidermis. The epidermal melanocytes do not increase in number, but extrude more pigment than normal into the basal epidermis.
- Most common in fair-skinned individuals
- May darken with sunlight exposure
- Typically located in malar region, but can be found anywhere on the face and neck (Figure 4)
- No treatment is necessary; can apply melanin-bleaching topical products
- Benign nevus (1–3 mm)- common lesions that arise from nevus cells, which are incompletely differentiated melanocytes found in clumps within the epidermis, dermis, and junction zone between these layers.
- Usually occur in childhood and increase at puberty
- Typically arise as flat, pigmented macules in childhood, representing junctional nevi
- Evolve into elevated/dome-like, pigmented compound nevi as they extend up into the epidermis and down into the dermis from the junctional zone
- In adulthood, the pigmented epidermal component involutes, resulting in a non-pigmented, amelanotic, dermal nevus (Figure 5)
- Frequently located on the eyelid margin, with typical appearance of molding to the globe along its posterior surface
- Malignant transformation of a junctional or compound nevus may occur, although rare
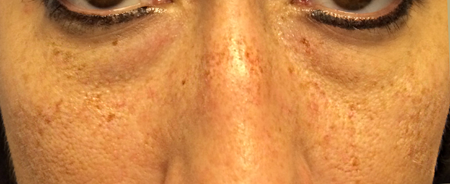
Figure 4. Differential diagnosis. Benign freckles, or ephelides, of the cheeks and nose demonstrating discrete, small, pigmented macules.
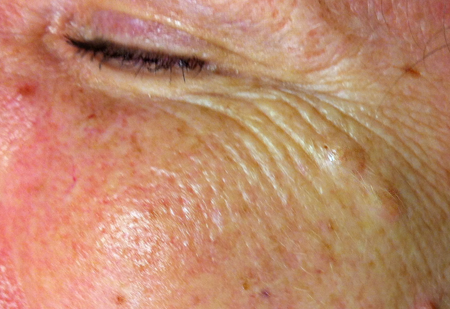
Figure 5. Differential diagnosis. Two dermal nevi are seen along the lateral crow’s feet as elevated lesions, larger than ephelides. Pigmentation of the epidermal component fades with age, resulting in relatively amelanotic papules.
- Simple lentigines (lentigo simplex)- small flat, pigmented lesions slightly larger in diameter than freckles (but less than 3mm), that are not associated with sun exposure
- Increased number of uniformly dispersed single melanocytes without aytpia, as well as variable melanin located in the basal keratinocytes
- Eyelid simple lentigines may be associated with autosomal dominant polyposis of the gastrointestinal tract (Peutz-Jeghers syndrome)
- Solar lentigines (solar lentigo)- slightly larger, 3–5 mm, uniformly pigmented lesions in sun exposed areas due to increased numbers of melanocytes
- Often increase with age and sun exposure due to ultraviolet-induced mutations leading to enhanced melanin production and abnormal pigment retention by keratinocytes
- Uniformly hyperpigmented, and slightly larger than simple lentigines
- Most frequently located on forehead, jawline, décolletage, dorsal hands and arms (Figures 6 and 7)
- Histologically may also demonstrate a bulb-like elongation of rete ridges that form a reticular pattern due to interconnections between adjacent strands
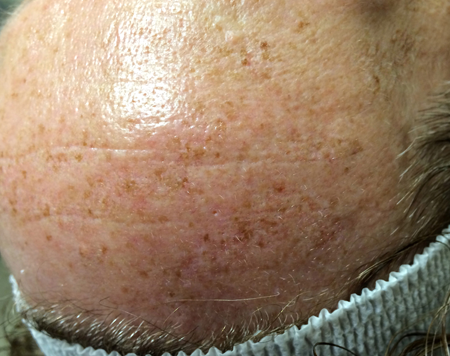
Figure 6. Differential diagnosis. Multiple flat, pigmented macules on the forehead and pretrichial skin, consistent with solar lentigines.
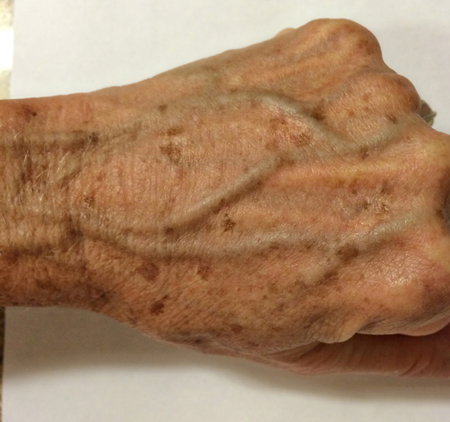
Figure 7. Differential diagnosis. Multiple flat, uniformly pigmented macules on the thin skin of the dorsal hand, consistent with solar lentigines.
- Melasma: flat hyperpigmentation typically occurring on the forehead or periocular skin in women during or after pregnancy or with a history of oral contraceptive use, chronic dermatitis. or rosacea
- Seborrheic keratosis
- Lichen planus-like keratosis
- Pigmented actinic keratosis
Patient management: treatment and follow-up
The Cancer Council of Australia published lentigo maligna guidelines in 2019 demonstrating poor quality evidence for most management. Standard of care: surgical excision with 5-10 mm margins. “Slow Mohs” (Mohs excision with formal, paraffin-embedded histopathologic analysis between stages) requires a longer duration of surgery over many days, but provides the benefit of potentially smaller surgical excision. If available, confocal microscopy may help in margin assessment. If excision not undertaken, radiotherapy is 2nd line and topical imiquimod 3rd line. Cryotherapy and laser therapy are not recommended as treatments.
Natural history
- Precancerous
- Lentigo maligna can be present for long periods (5–15 years) before invasion occurs, although rapid progression within months has been described
- Estimates of the lifetime risk of developing lentigo maligna melanoma in patients diagnosed with lentigo maligna approximately 2.2% to 5%
Medical therapy
- Topical chemotherapeutic agents
- Imiquimod 5% (Aldara) applied topically daily for 7–14 weeks. It is a topical immunomodulator that generates a local cytotoxic response. A local inflammatory reaction is a strong predictor of therapeutic benefit (Powell. Br J Dermatol 2009)
- Evaluation of inflammatory infiltrate following imiquimod treatment demonstrates T-helper lymphocytes mixed with a significant number of cytotoxic cells and monocytes or macrophages, suggesting a cytotoxic T-cell–mediated immune response.
- May be considered for monotherapy of lesions that are large or pose cosmetic challenges, although probably lower efficacy than surgery
- Ahmed et al. (Br J Dermatol 2000) described erythema and erosions arising in the treated areas 2–4 weeks after start of therapy, with time to complete clearing occurring from 5 to 13 weeks based on both clinical and histopathologic findings.
- Powell et al. (Clin Exp Dermatol 2004) first reported 10 of 12 patients with histopath cure of lentigo maligna using imiquimod three times a week for 6 weeks, although benign dermal pigmentation sometimes persisted.
- Kirtschig et al. (Acta Derm Venereol 2014) demonstrated lasting clearance of lentigo maligna in 23/24 patient after an average of 14 weeks of daily imiquimod
- Sometimes used as adjunct before or after surgical excision to decrease recurrence rates
- May also have a role in management when surgery is contraindicated
- Best for those incapable of undergoing surgery, recurrent lesions in which margins are difficult to clear/would cause disfigurement or functional impairment. Requires close follow up for recurrence for at least a decade (and will generally need skin checks given history). However, there is likely some continued active benefit for up to 6 months after treatment cessation.
- In the periocular region, Imiquimod (prescribed in a variety of protocols) shows a wide range of 56-86% complete treatment response.
- Chemotherapy
- Interferon, interleukin 2
- Possible observation for small macular lesions
Radiation
- First described by Miescher in 1954
- Ultrasoft xray/grenz-ray radiation:
- Grenz rays are very superficial RT (typically 10–30 kV), which only penetrate to the epidermal–dermal interface (Fogarty 2014)
- Radiation therapy typically fractionated, with the total dose divided into parts and given over daily treatments. The main benefit of fractionation is to take advantage of the difference in repair capacity between normal cells and tumor cells. Whereas normal cells within the radiation treatment volume are able to repair the radiation damage, tumor cells cannot and therefore die.
- Standard margins cannot be achieved and is a limitation of radiation
- Primary risk of using radiation therapy for lentigo maligna is the potential of missing a focal area of lentigo maligna melanoma
- Radiation therapy has been used as definitive treatment for LM, or as adjuvant therapy following excision
- Although more recent studies have demonstrated good results with radiation for treatment of LM and LMM, as well as good cosmetic outcomes, it is generally considered for patients who are elderly or may not be ideal candidates for surgery
- Hedblad et al. (J Am Acad Dermatol 2012) reported a large series of 593 patients treated with Grenz radiation therapy for LM/early LMM between 1990 and 2009. 350 were treated as primary therapy, with the rest treated in conjunction with excision or as recurrence-prophylactic treatment.
- Treatment was given twice a week over 3 consecutive weeks in total doses of 100 to 160 Gy
- Follow-up was up to 5 years in 241/593 patients
- 520 of 593 patients (88%) showed complete clearance after one fractionated treatment
- 53 of 593 patients (9%) relapsed within 2 years
- Recurrences after radiation therapy occur typically 2–4 years after treatment (Farshad, Br J Dermatol 2002)
- Increased incidence of other skin cancers following radiation treatment may occur
Surgery
- Surgery and complete excision remains the standard of care, although other nonsurgical options have arisen in popularity
- Standard excisions have a recurrence rate of 8%–20%
- Mapped serial excision: utilizing permanent sections and modified Mohs micrographic surgery has been studied in the periocular region
- Malhotra et al. (Ophthalmology 2003) studied perioocular lentigo maligna and lentigo maligna melanoma and reported 3% recurrence in 135 patients using mapped serial excision. They concluded that 5–10-mm margins are insufficient for complete removal of lentigo maligna and lentigo maligna melanoma
- Staged “Square Procedure” is useful for ill-defined lesions and for lesions with a high recurrence rate due to subclinical spread
- First described by Johnson et al. (J Am Acad Dermatol 1997) using a two-bladed knife to excise strips of skin in a square (or other polygon) around lentigo maligna in a staged fashion to determine clean margins prior to definitive excision and reconstruction.
- Cure achieved in 20/21 patients reported by Patel et al. (Clin Exp Dermatol 2014)
- Can result in large disfiguring excisions, especially if there are peripheral or adjacent regions of melanocytic hyperplasia with atypia that worry the dermatopathologist or surgeon
- Therefore it is important to note that the risk of progression of melanocytic hyperplasia to lentigo maligna or melanoma is unknown
- As noted above, surgical excision with 5-mm margins may be inadequate for lentigo maligna on sun-damaged skin where a background of melanocytic hyperplasia obscure the true borders of the lesion (Agarwal-Antal, J Am Acad Dermatol 2002)
- Optimal margin of excision is still controversial, with reports of 5mm to 2cm recommended
- Close observation for recurrence and onset of new lesions
Other management considerations
- In vivo reflectance confocal microscopy (RCM) can be used to assess treatment response to non-surgical therapy
- Characteristic RCM features of facial lentigo maligna include: a focal increase of atypical melanocytes and nests surrounding adnexal openings, sheets of mainly dendritic melanocytes, cord-like rete ridges at the dermoepidermal junction (DEJ), infiltration and/or pagetoid spread of adnexal structures by atypical melanocytes, cord-like rete ridges at the DEJ, infiltration of adnexal structures by atypical melanocytes, and epidermal disarray. (Ahlgrimm-Seiss, Br J Dermatol 2009)
- Guitera et al. (JAMA Dermatol 2013) demonstrated that RCM changed the management of 73% of patients with lentigo maligna
- Cryotherapy is not a recommended standard for lentigo maligna (although it is used for conjunctival disease), but may be useful for patients unable to undergo surgery. There is inadequate evidence to determine whether it may have a role as an adjunctive treatment to surgery. Imiquimod may be more useful.
- Temperatures of -4°C to -7°C selectively destroy melanocytes while preserving keratinocytes in skin and mucosal epithelia
Common treatment responses, follow-up strategies
- Instruction on self-examination for recurrence or new lesions
- Limit sun exposure (hat, topical sun block)
- Routine periodic follow-up evaluations
- Recurrence rate is high enough to warrant serial examination and followup
Preventing and managing treatment complications
- Ensure complete excision in the likely event that surgery is used for treatment
- Compromise of the functional integrity of the eyelid (cicatricial ectropion, lagophthalmos, ocular exposure)
- Additional surgery if indicated
- Lubrication, tarsorrhaphy
- Scarring
- Minimize by placement of incisions in normal relaxed skin tension lines, and avoiding tension on wound edges
- Allow scars to mature for 6–12 months at minimum
- Topical scar management (vitamin E, silicone gel or sheet, cortisone)
- Steroid injection
- Laser resurfacing options may minimize residual scarring
Disease-related complications
- Progression into invasive melanoma with or without distant metastasis
Historical perspective
- Lentigo maligna first described in 1890 (Hutchinson J)
- Radiation described in treatment of lentigo maligna in 1954 (Miescher J)
- Obtaining clear margins for resection: Standard recommendation of 5-mm margins was adequate in only 42% of cases (Agarwal-Antal, J Am Acad Dermatol 2002)
References and additional resources
- AAO, Basic and Clinical Science Course. Section 7: Orbit, Eyelids, and Lacrimal System, 2013-2014.
- AAO Monograph 8, Surgery of the Eyelids, Lacrimal System, & Orbit, 2nd edition, 2011.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol 2002;47(5):743-8.
- Ahlgrimm-Seiss V et al. Reflectance confocal microscopy of facial lentigo maligna and lentigo maligna melanoma: a preliminary study. Br J Dermatol 2009;161:1307-16.
- Ahmed I, Berth-Jones J. Imiquimod: a novel treatment for lentigo maligna. Br J Dermatol 2000; 143843- 845.
- Beltraminelli H, Shabrawi-Caelen LE, Kerl H, Cerroni L. Melan-A-positive ”pseudomelanocytic nests”: a pitfall in the histopathologic and immunohistochemical diagnosis of pigmented lesions on sundamaged skin. Am J Dermatopathol 2009; 31: 305.
- Blessing K, Sanders DS, Grant JJ. Comparison of immunohistochemical staining of the novel antibody melan-A with S100 protein and HMB-45 in malignant melanoma and melanoma variants. Histopathology 1998; 32: 139.
- Busam, K.J., et al., Expression of melan-A (MART1) in benign melanocytic nevi and primary cutaneous malignant melanoma. Am J Surg Pathol, 1998. 22: p. 976-82.
- Cohen, L.M., Lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol, 1995. 33: p. 923-36; quiz 937-40.
- El Shabrawi-Caelen L, Kerl H, Cerroni L. Melan-A: not a helpful marker in distinction between melanoma in situ on sun-damaged skin and pigmented actinic keratosis. Am J Dermatopathol 2004; 26: 364.
- Farshad A, Burg G, Panizzon R, et al. A retrospective study of 150 patients with lentigo maligna and lentigo maligna melanoma and the efficacy of radiotherapy using Grenz or soft X-rays. Br J Dermatol 2002; 146:1042–6.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for Lentigo Maligna: A Literature Review and Recommendations for Treatment. British J Dermatology 2014;170:52-8.
- Goldstein AM, Dracopoli NC, Engelstein M, et al. Linkage of cutaneous malignant melanoma/dysplastic nevi to chromosome 9p, and evidence for genetic heterogeneity. Am J Hum Genet 1994; 54:489-96.
- Guitera P et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscropy. JAMA Dermatol 2013:149:692-8.
- Guitera P, Waddell A, Paton E, Fogarty GB, Hong A, Scolyer RA, Stretch JR, O’Donnell BA, Pellacani G. A practical guide on the use of imiquimod cream to treat lentigo maligna. Australas J Dermatol. 2021 Nov;62(4):478-485. doi: 10.1111/ajd.13720. Epub 2021 Sep 16. PMID: 34529272.
- Hedblad MA, Mallbris L. Grenz ray treatment of lentigo maligna and early lentigo maligna melanoma. J Am Acad Dermatol 2012; 67:60-8.
- Hillesheim PB, Slone S, Kelley D, Malone J, Bahrami S. An immunohistochemical comparison between MiTF and MART-1 with Azure blue counterstaining in the setting of solar lentigo and melanoma in situ. J Cutan Pathol 2011; 38: 565.
- Hutchinson J. Notes on the cancerous process and on new growths in general. Arch Surg (London) 1890; 283- 86.
- Hutchinson J. Senile freckle with deep staining – a superficial epithelioma of the cheek. Arch Surgery (London) 1892; 3:159.
- Johnson TM et al. Usefulness of the staged excision for lentigo maligna and lentigo maligna melanoma: the “square” procedure. J Am Acad Dermatol 1997;37:758-64.
- Kim J, Taube JM, McCalmont TH, Glusac EJ. Quantitative comparison of MiTF, Melan-A, HMB-45 and Mel-5 in solar lentigines and melanoma in situ. J Cutan Pathol 2011;38: 775-9.
- King R, Googe PB, Weilbaecher KN, Mihm MC Jr, Fisher DE. Microphthalmia transcription factor expression in cutaneous benign, malignant melanocytic, and nonmelanocytic tumors. Am J Surg Pathol 2001; 25: 51.
- Kirtschig G et al. Twelve-week treatment of lentigo maligna with imiquimod results in a high and sustained clearance rate. Acta Derm Venereol 2014 Apr 3 [Epub ahead of print]
- Liu V, Mihm MC. Pathology of malignant melanoma. Surg Clin North Am 2003; 83(1):31-60.
- Ly L, Kelly JW, O’Keefe R, et al. Efficacy of imiquimod cream, 5%, for lentigo maligna after complete excision: A study of 43 patients. Arch Dermatol 2011; 147(10):1191-5.
- Malhotra R et al. Mapped serial excision for periocular lentigo maligna and lentigo maligna melanoma. Ophthalmology 2003;110:2011-18.
- McLeod M, Choudhary S, Giannakakis G, et al. Surgical treatments for lentigo maligna: a review. Dermatol Surg 2011; 37(9):1210-28.
- Neumann I, Patalay R, Kaushik M, Timlin H, Daniel C. Treatment of periocular lentigo maligna with topical 5% Imiquimod: a review. Eye (Lond). 2023 Feb;37(3):408-414. doi: 10.1038/s41433-022-02165-5. Epub 2022 Jul 14. PMID: 35835989; PMCID: PMC9905524.
- Patel AN et al. Johnson square procedure for lentigo maligna and lentigo maligna melanoma. Clin Exp Dermatol 2014:39:570-6.
- Powell AM et al. Imiquimod and lentigo maligna: a search for prognostic features in a clinicopathological study with long-term follow-up. Br J Dermatol 2009;160:994-8.
- Powell AM et al. Topical imiquimod immunotherapy in the management of lentigo maligna. Clin Exp Dermatol 2004;29:15-21.
- Powell AM, Robson AM, Russell-Jones R, et al. Imiquimod and lentigo maligna: a search for prognostic features in a clinicopathological study with long-term follow-up. British J Dermatology 2009; 160(5): 994-8.
- Robinson M, Primiero C, Guitera P, Hong A, Scolyer RA, Stretch JR, Strutton G, Thompson JF, Soyer HP. Evidence-Based Clinical Practice Guidelines for the Management of Patients with Lentigo Maligna. Dermatology. 2020;236(2):111-116. doi: 10.1159/000502470. Epub 2019 Oct 22. PMID: 31639788.
- Swetter SM et al. Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990-2000. J Invest Dermatol 2005;125:685-91.
- Weinstock MA, Sober AJ. The risk of progression from lentigo maligna to lentigo maligna melanoma. Br J Dermatol 1987;116:303-10.
- Wolf IH, Smolle J, Binder B, et al. Topical imiquimod in the treatment of metastatic melanoma to skin. Arch Dermatol 2003; 139273- 276.
