Microcystic Adnexal Carcinoma
Updated May 2024
Establishing the diagnosis
Etiology
- Microcystic adnexal carcinoma is a low grade sweat gland carcinoma.
- Other names for this entity include malignant syringoma, and sclerosing sweat gland carcinoma.
- This tumor develops on the central face, including the eyelids.
- Eccrine carcinomas, including microcystic adnexal carcinoma, express follicular stem-cell markers, suggesting origin from the folliculosebaceous apocrine unit (Mahalingam, Am J Dermatopathol 2010).
Epidemiology
- Around 700 cases of microcystic adnexal carcinoma have been reported in the literature.
- The initial six cases were all white patients, five were women and the average age was 44 years old (Goldstein, Cancer 1982).
- The tumor is rare in African Americans (Peterson, J Am Acad Dermatol 2001).
- In a Surveillance, Epidemiology and End Results (SEER) database analysis, 223 patients with MAC at all sites were identified indicating that it is a rare tumor (Yu, Am J Clin Oncol 2010).
- More than 50 cases of eyelid involvement with MAC have been described in the literature (Liyanage, Arch Ophthalmol 2010).
- A review of 93 patients compiled from the literature showed an average age of 56 (OPRS 2023:39:533)
History
- Eyelid microcystic adnexal carcinoma can present as a lid nodule, usually yellow or flesh colored, as lid thickening or as progressive lid distortion.
- The course is insidious and symptoms may be present for several years before the diagnosis is made.
Clinical features
- The clinical appearance of a microcystic adnexal carcinoma is of an erythematous plaque or papule.
- A brownish-gray mass with ulceration is typical.
- The typical distribution is in sun exposed areas.
- A recent literature review demonstrated the eyebrow and lower eyelid were the most common periocular sites of distribution (OPRS 2023:39:533).
- Microcystic adnexal carcinoma is typically aggressive locally with invasion but infrequently disseminates beyond the primary site (Pugh, Head Neck 2012).
- Metastasis to regional lymphatics is rare.
- There is a case report of a primary orbital microcystic adnexal carcinoma which presented with diplopia and enophthalmos (Wu-Chen, J Neuroophthalmol 2011).
- Secondary orbital invasion by an eyelid MAC has been reported several times (Marshall, Orbit 2003; Hoppenreijs, Br J Ophthalmol 1997)
- Congenital microcystic adnexal carcinoma has been reported at non-ocular sites (Fu, Arch Dermatol 2011; Smart, Pediatr Dermatol 2011).
- In the SEER analysis described above only 1% of tumors proved to have regional lymph node involvement.
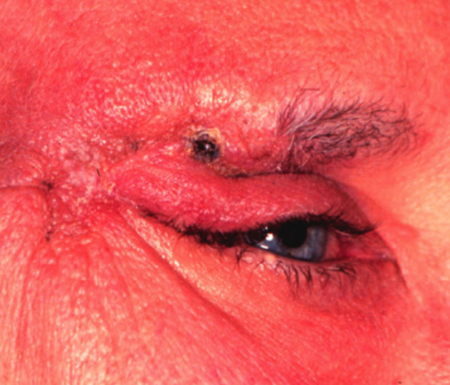
Figure 1. Microcystic adnexal carcinoma. Image courtesy Mark J. Lucarelli, MD.
Testing
- A recent literature review demonstrated a high rate of initial misdiagnosis, with only 36% accurate diagnosis on initial biopsy (OPRS 2023:39:533).
- The histopathology of microcystic adnexal carcinoma demonstrates islands of basaloid keratinocytes, some of which contain horn cysts and abortive follicles, embedded in a desmoplastic stroma.
- Ducts and gland-like structures lined by two-cell layers are present.
- The pathology is unusual in that deep invasion in the soft tissue, through the muscle layer, and perineural invasion are common but cytologic atypia and mitotic figures are rare.
- Immunohistochemistry can be helpful in differentiating between a morpheaform basal cell carcinoma, which characteristically is BerEP4 positive and microcystic adnexal carcinoma which characteristically is not (Seltheyer, J Cutan Pathol 2013).
- The monoclonal antibody BerEP4 is an epithelial marker that recognizes two glycopolypeptides (34 and 39) and also differentiates with a high degree of reliability between BCC and cutaneous squamous cell carcinoma.
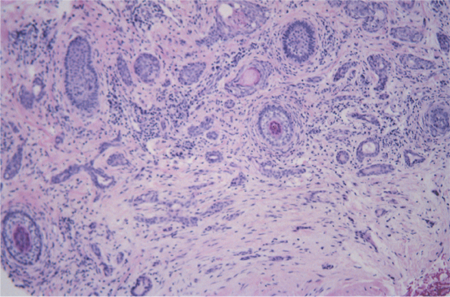
Figure 2. Microcystic adnexal carcinoma pathology. Image courtesy Mark J. Lucarelli, MD.
Testing for staging, fundamental impairment
- There have been no studies on staging of eyelid MAC
Risk factors
Microcystic adnexal carcinoma has been reported to arise in the context of immunosuppression and prior radiotherapy.
Differential diagnosis
- Basal cell carcinoma
- Syringoma
- Desmoplastic trichoepithelioma
- Sebaceous cell carcinoma
Patient management: treatment and follow-up
Natural history
- Microcystic adnexal carcinoma typically is deeply invasive local because it eludes clinical recognition for a long time, often years.
Medical therapy
- There are no reported medical treatments for microcystic adnexal carcinoma.
Radiation
- Of all primary periocular sweat gland carcinomas, microcystic adnexal carcinoma is likely the most aggressive (Baker MS, et al). Cases with perineural invasion have high risk of local recurrence — consider radiation in those cases, as well as any case of recurrence after excision (Baker MS, et al).
- Radiation therapy for facial microcystic adnexal carcinoma has been described as primary and treatment (Pugh, Head Neck 2012).
- Some have questioned the adequacy of radiotherapy as primary treatment although it has the advantage of avoiding disfiguring surgery (Baxi, J Med Imaging Rad Oncol 2010).
- Ocular morbidity with radiotherapy can be significant and the usual total dose is 50-60 Gray, delivered in standard fractionation.
Surgery
- Literature review demonstrates a variety of surgical management methods: surgical excision with margins or frozen section, Mohs micrographic surgery (OPRS 2023:39:533).
Other management considerations
- None.
Common treatment responses, follow-up strategies
- Local recurrence is common with microcystic adnexal tumor, due to the deep invasive nature of the tumor.
- With adequate tumor excision the recurrence rate is low after surgical excision, without adjuvant radiotherapy (Leibovitch, Ophthalmologica 2006).
Preventing and managing treatment complications
Morbidity for these tumors is related to the extent of tumor involvement, the need for surgical excision and effects of radiotherapy when used.
Disease-related complications
In the SEER database analysis described above for MAC the overall 10 year survival was 86%.
- However, there is only a single case in the literature of tumor related death due to microcystic adnexal carcinoma (Ohta, Dermatol Surg 2004).
- There are rare cases of systemic metastases; the survival outcome for some is not known (Eisen, Dermatol Surg 2005).
Historical perspective
Microcystic adnexal carcinoma was first described by Goldstein in 1982 (Goldstein, Cancer 1982).
References and additional resources
- Baker MS, Yin VT, Ivan D, Allen RC, Carter KD, Esmaeli B, Shriver EM. Epidemiology and Prognosis of Primary Periocular Sweat Gland Carcinomas. Ophthalmic Plast Reconstr Surg. 2017 Mar/Apr;33(2):101-105. doi: 10.1097/IOP.0000000000000658. PMID: 26974419.
- Baxi S et al. Microcystic adnexal carcinoma of the skin: the role of adjuvant radiotherapy. J Med Imaging Rad Oncol 2010; 54:477-82.
- Cheung J, Rabinowitz MP, Tuluc M, Milman T. Periocular Microcystic Adnexal Carcinoma: A Case Report and a Major Review. Ophthalmic Plast Reconstr Surg. 2023 Nov-Dec 01;39(6):533-541. doi: 10.1097/IOP.0000000000002419. Epub 2023 Jun 6. PMID: 37279021.
- Clement CI, Genge J, O’Donnell BA, Lochhead AG: Orbital and periorbital microcystic adnexal carcinoma. Ophthal Plast Reconstr Surg 2005; 21:97.
- Diamantis SA, Marks VJ. Mohs micrographic surgery in the treatment of microcystic adnexal carcinoma. Dermatol Clin 2011; 20:185-90.
- Eisen DB, Zloty D. Microcystic adnexal carcinoma involving a large portion of the face: when is surgery not reasonable? Dermatol Surg 2005;31:1472-7.
- Fu T et al. Congenital microcystic adnexal carcinoma. Arch Dermatol 2011; 147:256-7.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer 1982; 50:566.
- Hoppenreijs VP, Reuser TT, Mooy CM, de Keizer RJ, Mourits MP. Syringomatous carcinoma of the eyelid and orbit: a clinical and histopathological challenge. Br J Ophthalmol 1997;81:668.
- Liyanage SE et al. Delayed diagnosis of microcystic adnexal carcinoma in progressive eyelid distortion. Arch Ophthalmol 2010;128:132-5.
- Leibovitch I, Hulligol SC, Richards S, et al: Periocular microcystic adnexal carcinoma: management and outcome with Mohs’ micrographic surgery. Ophthalmologica 2006; 220:109.
- Mahalingam M et al. Expression of stem-cell markers (cytokeratin 15 and nestin) in primary adnexal neoplasms-clues to etiopathogenesis. Am J Dermatopathol 2010; 32:774-9.
- Marshall J, Mortimore R, Sullivan T. Sclerosing sweat duct carcinoma of the orbit. Orbit. 2003; 22:165.
- Ohta M et al. Metastatic microcystic adnexal carcinoma: an autopsy case. Dermatol Surg 2005;30:957-60.
- Ong T et al. Microcystic adnexal carcinoma of the eyebrow. OPRS 2004;20:122-5.
- Peterson CM, Ratz JL, Sangueza OP: Microcystic adnexal carcinoma: First reported case in an African American man. J Am Acad Dermatol 2001; 45:283.
- Pugh TJ, Lee NY, Pacheco T, Raben D: Microcystic adnexal carcinoma of the face treated with radiation therapy: a case report and review of the literature. Head Neck 2012; 34;1045.
- Seltheyer K, Nelson P, Kutzner H, Patel RM: The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cut Pathol 2013; 40:363.
- Smart DR et al. Microcystic adnexal carcinoma: the first reported congenital case. Pediatr Dermatol 2011;28:35-8.
- Wu-Chen, WY et al. Unusual presentation of primary orbital microcystic adnexal carcinoma. J Neuroophthalmol 2011;31:147-50.
- Yugueros P, Kane WJ, Goellner JR: Sweat gland carcinoma: a clinicopathologic analysis of an expanded series in a single institution. Plast Reconstr Surg 1998;102:705.
- Yu JB et al. Surveillance, Epidemiology, and End Results (SEER) database analysis of microcystic adnexal carcinoma (sclerosing sweat duct carcinoma) of the skin. Am J Clin Oncol 2010;33:125-7.
