Methicillin-Resistant Staphylococcus Aureus (MRSA)
Updated July 2024
Establishing the diagnosis
Etiology
- Mutated and selected, more virulent and resistant to beta-lactam antibiotics
- Includes resistance to methicillin, penicillin, and cephalosporins
- Susceptible hosts are both immunocompetent and immune compromised
- Invades intact skin or can enter through local injury
- Colonizes conjunctiva and lid margin
- Colonizes and infects obstructed lacrimal system
- Can invade orbit via conjunctiva (Arch Ophthalmol 2009; 127:941)
- Can infect lacrimal gland directly or hematogenously
- Genotype USA300 is the major community associated strain in most of the US (Ophthalmology 2006; 113:1455).
- Healthcare-associated MRSA strain is USA100
- Community-acquired MRSA (CAMRSA) differs from healthcare-associated MRSA.
- CAMRSA is more easily transmitted through close contact.
- CAMRSA is less likely to be multiply drug resistant to non-beta lactam antibiotics.
- CAMRSA has a higher prevalence of genes for exotoxins (i.e., Panton-Valentine leukocidin) that promote abscess formation.
Epidemiology
- Study from Kaiser Healthcare of 137 culture-positive ophthalmic MRSA patients age 0–18 years 2002–2008 (J Am Acad Ped Ophthalmol Strab 2013; 17:243)
- 58% community acquired
- Conjunctivitis 40%, chalazion 25%, orbital cellulitis 19%, dacryocystitis 11%, brow abscess 3%
- Study of 112 Veterans Affairs Medical Centers 2007–2010, MRSA admission prevalence was 11.4% (Clin Infect Dis 2014; 58:32)
- Among residents of long-term care facilities in Hawaii the prevalence of MRSA was 35% in 2000, increased to 58% in 2005 (Hawaii Med J 2010; 69:126).
- Fingertips of 523 healthcare workers exposed to patients with MRSA were culture positive in 5% (J Hosp Inf 2010; 75:107).
- Fingers were culture-positive even if exposure was to patient environment, not direct contact.
- Slightly reduced, but still present, in those who used alcohol hand rub (3%), or washed with soap (3%)
- Decolonization study in Switzerland 2007–2009 of every patient with newly detected MRSA: colonization or infection (Infection 2013; 41:33)
- Regimen was nasal mupirocin ointment for 5 days, chlorhexidine mouth rinse, and whole-body wash with didecyldimonium chloride.
- Success rate was only 65% at 13-month follow-up.
- Recurrence often due to respiratory tract nidus
- In most US cities MRSA is the most common pathogen cultured from patients with skin and soft-tissue infections (Exp Opin Pharmacother 2010; 11:3009).
- Skin and soft tissue infections represent 1% of patients presenting to an emergency department, 20% have abscesses requiring drainage.
History
- Skin lesions, often incorrectly attributed to insect bites
- Redness
- Swelling
- Pain, tenderness
- No specific time course indicative of MRSA
Clinical features
- In addition to cellulitis and abscess, MRSA can cause necrotizing fasciitis.
- Fluctuance can indicate abscess (Figure 1)
- Deep abscess can be obscured by overlying cellulitis
- Ultrasound can identify deep anechoic space
- Needle aspiration can identify deep purulence
- Absence of purulence on needle aspiration might be due to location or high viscosity and does not rule out abscess (NEJM 2014; 370:11)
- Acute bacterial dacryoadenitis a unique clinical presentation of MRSA (BJO 2013; 97:735)
- Among 15 cases of MRSA orbital cellulitis, 5 had lacrimal gland abscess or dacryoadenitis (Ophthalmology 2012; 119:1238)
- In addition to eyelid swelling, redness, ptosis, frequently diplopia, limitation of eye movement
- CT evidence of lacrimal gland swelling
- Indentation of the globe by an edematous lacrimal gland is common.
- In 11 cases, average time to resolution was 9 days (BJO 2013; 97:735).
- Intravenous antibiotics often needed (Arch Ophthalmol 2014; 132:993)
- Purulent fluid may bee seen in the superior fornix along with an S-shaped lid deformity. (Liu, JAMA Ophthalmol 2014).
- Atypical presentations of orbital cellulitis related to MRSA include lid swelling without and upper respiratory infection, lacrimal gland focus, multiple orbital abscess or lac of paranasal sinus disease (Mathias, Ophthalmology 2012)
- Intracranial extension can be reported (44% in 4/11 patients) (Ang, Int Ophthalmol, 2023)
- Meningitis is a rare presentation (Nair, J Pediatr Ophthalmol Strabismus, 2020)
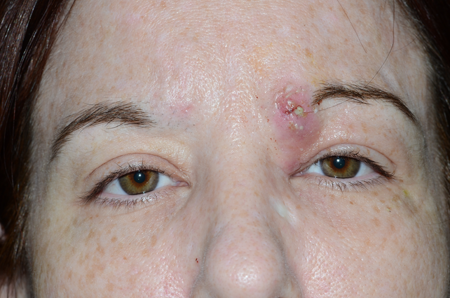
Figure 1. Abscess. Courtesy Scott M. Goldstein, MD.
Figure 2. MRSA infection. Courtesy Richard C. Allen, MD, PhD, FACS.
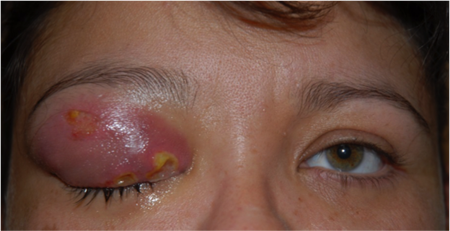
Figure 3. MRSA infection. Courtesy Richard C. Allen, MD, PhD, FACS.
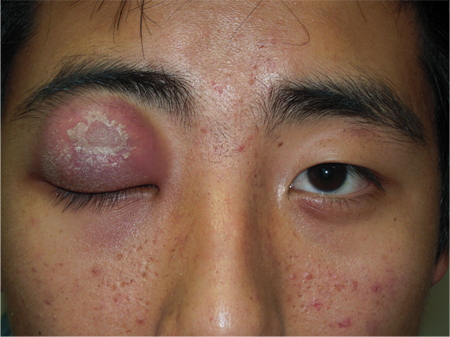
Figure 4. MRSA infection. Courtesy Rona Z. Silkiss, MD, FACS.
Testing
- Culture and sensitivities to confirm diagnosis (Ophthalmology 2006; 113:1455)
- Routine culture of soft tissue infection was less critical prior to the emergence of CAMRSA
- Only two studies have evaluated the use of MRSA PCR testing in cases of pediatric orbital cellulitis. There is a 98% negative predicted value and overall lower use of anti-MRSA antibiotics without an increase in hospital readmission is seen (Hamilton, J Pediatric Infect Dis Soc, 2024).
- No staging
Risk factors
- Atopic dermatitis predisposes to S aureus colonization of the eyelid margin and conjunctival fornix (Ophthalmology 2000; 107:2167).
- Suspect S aureus if periocular infection develops after eye surgery (e.g., scleral buckle for retinal detachment) in a patient with atopic dermatitis (Ophthalmology 1999; 106:142).
- In a recent study of 15 patients with MRSA orbital cellulitis none had preceding upper respiratory infection (Ophthalmology 2012; 119:1238).
- One patient had antecedent eyelid trauma.
Differential diagnosis
- For unusual clinical infections such as purulent dacryoadenitis, MRSA is likely pathogen.
- For common infections such as dacryocystitis, likelihood of MRSA depends on regional prevalence and severity of infection.
- Other bacterial pathogens such as streptococcus
- Fungal infection
- Inflammatory lesion can be with necrosis (e.g., pyoderma gangrenosum).
- Spider bite
Patient management: treatment and follow-up
Natural history
- Abscess from MRSA infection is painful.
- Abscess drainage rated second-most painful procedure after nasogastric intubation in emergency department (Ann Emerg Med 1999; 33:652)
- Needle aspiration can help identify progression to abscess from cellulitis, but is significantly less likely to adequately treat an abscess compared to open drainage.
- High suspicions for the neonatal population
Medical therapy
- 95% of community acquired MRSA in the USA is sensitive in vitro to trimethoprim-sulfamethoxazole (TMP-SMX), clindamycin, and tetracycline. In contrast to healthcare-associated MRSA, community acquired MRSA is usually sensitive to clindamycin as well (JAMA 2003; 290:2976).
- Typically, Bactrim or Doxycycline are the first-line treatment for MRSA in 2015.
- Children younger than 8 should not be given Doxycycline because it can permanently stain tooth enamel.
- Bactrim has less effective for Streptococcus species.
- Oral dose for clindamycin is 300–450 mg 3 times daily for adults, 10–13 mg/kg 3–4 times a day for children.
- TMP-SMX (Bactrim) and tetracycline are often preferred over clindamycin for MRSA alone. If a polymicrobial infection is encountered, TMP and tetracycline might be inappropriate because Group-A strep is usually resistant.
- A first-generation cephalosporin can be added to TMP-SMX or tetracycline in place of clindamycin.
- Rifampin is highly active against susceptible community acquired MRSA, but frequent mutation to resistance precludes its use as a single agent (NEJM 2007; 357:4).
- Do not use fluoroquinolones to treat skin and soft tissue infection due to community-acquired MRSA.
- Prevalence of resistance is high
- Resistance develops rapidly
- Intravenous vancomycin is first-line treatment for hospitalized patients.
- Do not use intravenous clindamycin as sole therapy when the patient is moderately to severely ill (NEJM 2007;357:4).
- Intravenous TMP-SMX can be significantly less effective than vancomycin (Ann Int Med 1992; 117:390).
- Topical antibiotics can be appropriate for superficial infection.
- Bacitracin ophthalmic ointment
- Polysporin (bacitracin, neomycin, polymyxin)
- Gentamicin ophthalmic ointment
- Mupiricin ointment for non-ophthalmic locations
- Antibiotic therapy after draining an abscess might not be necessary.
- In 161 children with drained MRSA abscess and treated with trimethoprim-sulfamethoxazole (TMP-SMX), there was no difference in healing versus placebo (Ann Emerg Med 2010; 55:401).
- Similar result in placebo controlled study of 212 adults (Ann Emerg Med 2010; 56:283).
Surgery
- Primary treatment for an abscess is incision and drainage.
- Incision should be along a tension line, to minimize scarring.
- A single incision is preferable, long enough for introduction of instruments and to adequately drain infection.
- A 1-cm incision should be adequate (Acad Emerg Med 2013; 20:27).
- The benefit of irrigation has not been studied (NEJM 2014; 370:11).
- In a randomized study of 48 adults, packing produced more pain with similar healing rates compared with no packing (Acad Emerg Med 2009; 16:470).
- Wick or drain might be preferable to packing.
- Primary closure after drainage reduces healing time with similar healing rates (Am J Emerg Med 2011; 29:361).
- Primary closure is not recommended in a patient with systemic infection or predisposition such as diabetes.
- Risk of persistent loculation and focus of systemic disease outweigh local wound considerations.
Other management considerations
- Criteria for admission for intravenous antibiotics:
- Large abscess requires operating room drainage
- Systemic illness
- Age younger than 6 months
- Immunosuppression
Common treatment responses, follow-up strategies
- Most bacterial infections will improve significantly within 1–2 days after drainage of an abscess although it can take 1–2 weeks for the swelling to fully fade.
- Oral antibiotic therapy typically takes 24–48 hours to start working.
- If there is worsening or minimal response within 48 hours, there might be a deeper infection. Reassess to see if there is anything else that needs to be drained. Can add a second oral antibiotic or if worsening, consider IV antibiotic therapy.
- Duration of antibiotic therapy depends on clinical response and can be as brief as 10–14 days.
- Failure to respond to initial therapy can be addressed with inpatient IV treatment and/or surgery as above.
Preventing and managing treatment complications
Clindamycin risks include antibiotic associated diarrhea and Clostridium difficile pseudomembranous colitis.
Disease-related complications
- Instances of severe visual loss have been reported in recent case series (Ophthalmology 2006; 113:1455).
- MRSA induces necrosis with delayed treatment
- Bilateral blindness from community-associated MRSA orbital cellulitis was reported in an otherwise healthy 44-year-old man (AJO 2005; 140:740).
- The patient was incarcerated and developed fever and chills 2 days after squeezing a pustule on his nares.
- There were no additional risk factors such as HIV, diabetes, steroid use, or hospitalization.
- Initial treatment was with TMP-SMX and rifampin; 4 days later he was hospitalized for intravenous vancomycin, ceftriaxone, and metronidazole.
- By day 11 he progressed to bilateral no-light-perception and cavernous sinus thrombosis. Vision loss was due to retinal and optic-nerve infarction.
Historical perspective
- Methicillin resistance was first recognized shortly after the introduction of methicillin in the early 1960s (NEJM 2007;357:4).
- MRSA was confined to healthcare facilities and illicit drug users until the 1990s.
- Began to appear in the community with new strains (J Infect Dis 2002; 186:1344)
References and additional resources
- Ang T, Cameron C, Tong JY, Wilcsek G, Tan J, Patel S, Selva D. Methicillin-resistant Staphylococcus aureus-associated orbital cellulitis: a case series. Int Ophthalmol. 2023 Aug;43(8):2925-2933.
- Hamilton S, Taylor M, Schneider JG, Howe Z, Sharma M, Boyd K, Manaloor JJ, Chehab H, Espinel A, Hamdy RF, Wood JB. Assessing the Diagnostic Performance and Clinical Utility of Nasal Methicillin Resistant Staphylococcus aureus PCR Testing in Pediatric Orbital Cellulitis. J Pediatric Infect Dis Soc. 2024 Jun 14:piae061.
- Liu W, Rootman DB, Berry JL, Hwang CJ, Goldberg RA. Methicillin-resistant Staphylococcus aureus dacryoadenitis. JAMA Ophthalmol. 2014 Aug;132(8):993-5.
- Mathias MT, Horsley MB, Mawn LA, et al. Atypical presentations of orbital cellulitis caused by methicillin-resistant Staphylococcus aureus. Ophthalmology. 2012; 119:1238-1243.
- Nair AG, Rathi N, Apte MK, Marathe TR, Potdar NA, Shinde CA. Simultaneous Bilateral Orbital Cellulitis With Meningitis Caused by Methicillin-Resistant Staphylococcus aureus in an Immunocompetent Infant. J Pediatr Ophthalmol Strabismus. 2020 Jun 23;57:e34-e37.
- Reem RE, Van Balen J, Hoet AE, Cebulla CM. Screening and characterization of Staphylococcus aureus from ophthalmology clinic surfaces: A proposed surveillance tool. Am J Ophthalmol. 2014; 157:781-787.
- Liu W, Rootman DB, Berry JL, Hwang CJ, Goldberg RA. Methicillin-resistant Staphylococcus aureus dacryoadenitis. Arch Ophthalmol. 2014; 132:993-995.
