Paranasal Sinus Anatomy and Physiology
Updated May 2024
Raymond Cho, MD; Erik K. Weitzel, MD
Introduction
- Paranasal sinuses pneumatize facial bones
- Proposed functions: (Keir, J Laryngol Otology 2009)
- Humidify/warm inspired air
- Lighten facial bones
- Protect sensory organs and brain from trauma
- Impart resonance to voice
- Major source of nitric oxide for respiratory tract (Djupesland, Am J Otolaryngol 2001)
- Orbit bordered on 3 sides by sinuses
- Floor: maxillary
- Medial: ethmoid
- Roof: frontal
- Optic canal: sphenoid
- Sinus disease often affects the orbit
- Understanding of sinus anatomy is important in many orbital/lacrimal procedures and management of orbital trauma
Physiology
- Lined by pseudostratified, ciliated respiratory mucosa
- Goblet cells produce acidic mucin
- Cilia beat in coordinated pattern to move mucus toward ostia
- Concentration of cilia increases close to ostia
- Adequate sinus outflow essential to proper functioning
- Vascular supply from various branches of internal and external carotid arteries
- Olfaction from cranial nerve I
- Sensory innervation from V1 and V2
- Autonomic innervation:
- Sympathetic stimulation reduces nasal blood flow and decongests mucosa
- Nasal cycle: alternating congestion and decongestion every 0.5–3 hours
- Parasympathetic system innervates glands; stimulation leads to watery secretion
- Immunologic function
- Nostril hairs and mucus filter particles from inspired air
- Humoral and cellular responses
- Nitric oxide has antiviral/antimicrobial properties and up-regulates ciliary motility
Anatomy
Nasal cavity
- Divided in midline by nasal septum
- Vomer, perpendicular plate of ethmoid, maxillary crest, septal cartilage
- Superior/middle/inferior turbinates (possible supreme) occupy lateral wall
- Maxilla and palatine bone comprise floor
- Cribriform plate comprises roof
- Terminal branches of olfactory nerve (cranial nerve I) enter nose through cribriform plate
- Vascular supply: sphenopalatine artery (branch of maxillary artery), ethmoidal arteries, end branches of facial artery, ascending pharyngeal arteries
- All paranasal sinuses drain into nasal cavity
- Superior meatus: posterior ethmoid, sphenoid sinus
- Middle meatus: frontal sinus, maxillary sinus, anterior ethmoid
- Semilunar hiatus contains openings to first three, bordered by:
- Uncinate process (anterior/inferior)
- Ethmoid bulla (posterior/superior)
- Inferior meatus: nasolacrimal duct
Maxillary sinus
- Largest of paranasal sinuses (about 15 ml in volume)
- Present at birth, continues to expand until descent of secondary teeth
- Drains into middle meatus through maxillary ostium
- Roof of sinus forms floor of orbit.
- Infraorbital neurovascular bundle travels along roof of sinus
- Bone much thinner medial to infraorbital canal and prone to fracture
- Pterygopalatine fossa behind posterior sinus wall
- Nasolacrimal duct travels anteromedial to sinus.
Ethmoid sinus
- Present at birth and expands with growth
- Multiple, thin-walled air cells lie between medial orbital wall and lateral wall of nose.
- Can pneumatize into frontal, sphenoid, palatine, or lacrimal bones
- Divided into anterior and posterior components
- Anterior drains into middle meatus.
- Posterior drains into superior meatus.
- Ethmoid bulla: large anterior cell posterior to uncinate process
- Anatomic variants:
- Agger nasi: most anterior ethmoid air cell, intervenes between lacrimal sac fossa and middle turbinate
- Concha bullosa: pneumatization of middle turbinate
- Haller cell: pneumatization of medial orbital floor
- Onodi cell: sphenoethmoidal air cell
- Lamina papyracea forms medial wall of orbit.
- Roof of ethmoid sinus (fovea ethmoidalis) borders anterior cranial fossa.
- Anterior and posterior ethmoidal bundles enter sinus from orbit at level of fronto-ethmoid suture.
- Anterior ethmoidal foramen (AEF) about 24 mm behind anterior lacrimal crest
- Posterior ethmoidal foramen (PEF) about 12 mm behind AEF
- Ethmoidal nerves arise from nasociliary nerve (branch of V1).
Sphenoid sinus
- Evaginates from posterior nasal roof to pneumatize the sphenoid bone
- Rudimentary at birth; reaches full size at puberty
- Sinus divided in midline by bony septum
- Normally occupies body of sphenoid only
- Occasionally can pneumatize into sphenoid wings, pterygoid, and occipital bone
- Optic canal located superior and lateral to sinus wall
- Sella turcica lies above posterior roof of sphenoid sinus.
- Drains into posterior nasal cavity
Frontal sinus
- Develops from evaginations of frontal recess within frontal bone
- Absent at birth, pneumatization begins about age 6 and is complete by early adulthood.
- Size and shape extremely variable and can be asymmetric
- Up to 5% have agenesis on one or both sides.
- Separated by midline intersinus septum
- Sinus drains through frontonasal outflow tracts into anterior portion of middle meatus
Clinical correlations
Sinus infection
- Viral rhinosinusitis
- For first 10 days of rhinosinusitis, etiology is viral in 60%–90% of cases.
- Antibiotics are indicated only after day 10 of routine sinusitis symptoms or a “double worsening” of the symptoms during the first 10 days.
- Acute bacterial rhinosinusitis (ABRS)
- Can lead to preseptal cellulitis, orbital cellulitis, subperiosteal abscess, or orbital abscess (Bilyk, Curr Opin Ophthalmol 2007)
- Most common organisms: Staphylococcus, Streptococcus, H influenza
- Location important in guiding management of subperiosteal abscess (Garcia, Ophthalmology 2000)
- Ethmoid (most common): often responds to IV antibiotics, especially in younger children
- Subperiosteal abscess originating from other sinuses more likely to require surgical drainage
- Chronic rhinosinusitis (CRS)
- 12 weeks or more of sustained sinus symptoms, confirmed with radiographic or endoscopic evidence of sinus disease
- Divided broadly into polyp or non-polypoid sinusitis
- Considered more immunologic driven than ABRS
- Steroids are fundamental treatment modality.
- Antibiotics are used secondarily.
- Common contributing comorbidities: asthma, environmental allergies, tobacco use
- Fungal sinus disease
- Invasive fungal
- Potentially devastating condition; high risk of orbital involvement, vision loss, or death
- Occurs in immunocompromised patients
- Organisms
- Mucormycosis (Mucorales or Rhizopus) (Schwartz, Surv Ophthalmol 1977)
- Large non-septate hyphae
- Chelating agents helpful for treatment
- Aspergillus (Levin, Surv Ophthalmol 1996)
- Similar clinical picture to mucormycosis
- Chelating agents not helpful
- Treatment
- Debridement of involved tissue
- Antifungals: systemic and intraorbital
- Reversal of immunocompromised state
- Chronic invasive/granulomatous
- Endemic to Middle East
- Potentially vision threatening
- Much slower clinical progression than invasive fungal sinusitis
- Fungus ball
- Benign, does not involve mucosa
- Does not involve orbit
- Treatment is removal
- Allergic fungal (Chang, OPRS 2000)
- Complex interaction of allergy, bacterial biofilm, altered immune response, and genetic expression
- Can cause significant bony remodeling of orbit that self-corrects after functional endoscopic sinus surgery (FESS)
- Treatment is multimodal:
- Control of comorbidities
- Topical treatment of mucosa
- Immunosuppression
- Aggressive FESS
Tumors
- Benign
- Mucocele
- Commonly from frontal sinus
- Inverting papilloma
- Malignant transformation possible
- Fibrous dysplasia/ossifying fibroma
- Present during childhood
- Meningioma
- Malignant (Frazell, Cancer 1963)
- Squamous cell carcinoma
- Most common epithelial sinus malignancy (60%)
- 80% arise from maxillary sinus
- Adenoid cystic carcinoma, adenocarcinoma
- Aggressive, poor prognosis
- Lymphoma
- 90% B-cell origin
- Natural killer T-cell lymphoma: predilection for nasopharynx
- Also known as lethal midline granuloma (Parker, Am J Otolaryngol 2010)
- Esthesioneuroblastoma (olfactory neuroblastoma)
Inflammations
(Weber, Radiol Clin N Am 1987)
- Sarcoidosis
- Granulomatosis with polyangiitis (Wegener’s granulomatosis) (Harman, Surv Ophthalmol 1996)
- Necrotizing granulomatous vasculitis, highly destructive
- Can invade orbit from sinuses or involve orbit primarily
- Other sites of involvement: upper respiratory tract, lungs, kidneys
- Diagnosis: C-ANCA, biopsy
- Treatment: steroids, immunosuppressants
- Polyarteritis nodosa
- Churg Strauss disease
Trauma and structural abnormalities
- Orbital blowout fractures
- Floor and medial wall most commonly involved due to thinness
- Floor fractures most often occur medial to infraorbital canal
- Infraorbital hypoesthesia often associated with floor fractures
- Medial wall fractures often limited to a few ethmoid air cells
- Ethmoid-maxillary suture (inferomedial strut) provides strong structural support at the junction of the two sinuses (Figure 1)
- Fracture of strut requires significant energy
- Repair of floor and medial wall more difficult due to loss of implant support
- Often requires “wraparound” implant or single shaped implant to span entire defect (Cho, Craniomaxillofacial Trauma Reconstr 2013)
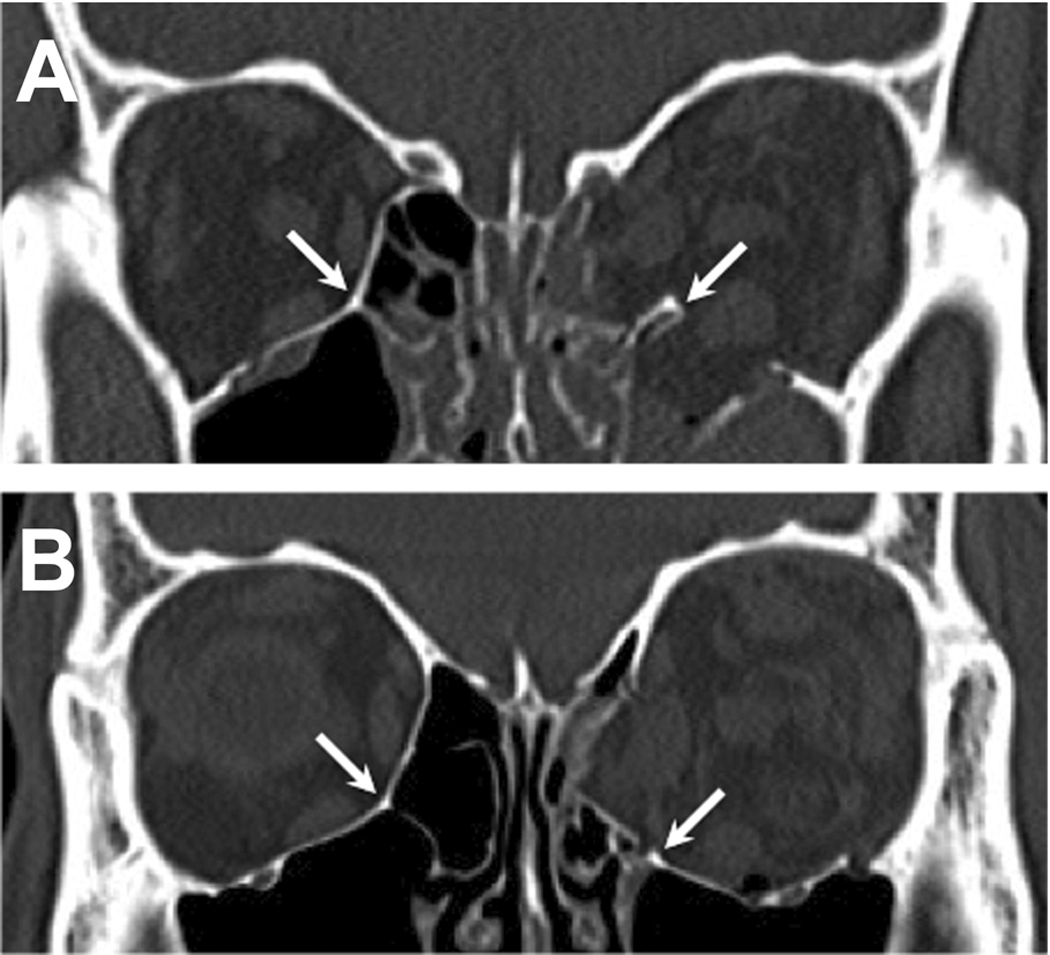
Figure 1. Combined left orbital floor and medial wall fractures (two separate cases). Inferomedial strut (white arrow) is intact in A, but fractured and inferiorly displaced in B. Reprinted (need permission) from Cho RI, Craniomaxillofac Trauma Reconstr 2013.
- Indications for repair (Burnstine, Ophthalmology 2002)
- Extraocular muscle entrapment (urgent)
- Diplopia within 30 degrees of primary gaze or visually significant
- Aesthetically unacceptable enophthalmos or globe malposition
- More likely to occur if fracture involves > 50% of surface area
- Frontal sinus fractures (Doonquah, Oral Maxillofacial Surg Clin N Am 2012)
- Frontal bone very strong, significant energy required to cause fracture
- Often associated with traumatic brain injury
- Potential sequelae:
- Frontal sinus outflow obstruction causing sinusitis and/or mucocele
- Traumatic orbital cephalocele (large orbital roof defect)
- Facial deformity
- Management controversial
- Observation
- Exenteration
- Cranialization
- Obliteration
- Traumatic optic neuropathy (Warner, Curr Opin Ophthalmol 2010)
- Typically caused by severe trauma, often associated with frontal sinus or orbital roof fractures
- Energy can be directed from forehead along orbital roof to optic canal (with or without fracture)
- Sphenoid fractures sometimes detectable on CT scan
- Treatment controversial
- High-dose steroids advocated by some
- Optic canal decompression can be attempted through trans-sphenoidal approach
- International Optic Nerve Trauma Study (IONTS) showed no definitive benefit from either high-dose steroids or optic nerve decompression
- Silent sinus syndrome (Annino, Curr Opin Otolaryngol Head Neck Surg 2008)
- Maxillary sinus atelectasis caused by impaired outflow through the ostiomeatal complex
- Chronic fluid accumulation and resorption causes negative pressure
- Collapse of orbital floor causes enophthalmos and globe dystopia
- CT findings: maxillary sinus hypoplasia and opacification, orbital expansion, lateralization of uncinate process
- Treatment
- Restoration of normal sinus outflow
- Enophthalmos/hypoglobus can spontaneously resolve after several months
- Consider orbital floor augmentation if malposition persists following period of observation
Surgery
Orbital decompression
(Kazim, Smith & Nesi’s Ophthalmic Plastic and Reconstructive Surgery 2012)
- Transantral decompression
- First described by Walsh and Ogura in 1957
- Removal of orbital floor and portion of medial wall/ethmoid sinus through Caldwell-Luc approach
- Largely abandoned due to high incidence of postoperative diplopia and globe dystopia
- Floor decompression (transconjunctival approach)
- Described by Hirsh in 1930, popularized by McCord in 1979
- Can be combined with medial decompression
- Medial decompression
- First reported by Sewall in 1936
- Endoscopic approach reported by Kennedy in 1990
- Transcaruncular approach described by Shorr in 2000
- Removal of lamina papyracea and ethmoid air cells
- Can be combined with partial or complete floor decompression
- Risk of diplopia and/or globe dystopia can be reduced by preservation of inferomedial strut
- Lateral decompression
- Described by Dollinger in 1911
- Deep bone removal popularized by Leone in 1989
- Removal of lateral wall
- Sinuses not involved unless greater sphenoid wing is pneumatized
- Results dependent on amount of bone removed from deep orbit
- Roof decompression
- Rarely performed due to risk to frontal sinus and brain
Lacrimal surgery
- Nasolacrimal duct (NLD) probing/stenting
- Familiarity with nasal anatomy important in locating probe in nose
- Retrieval device should be directed inferolaterally upon entry in the nares and run along the junction of the floor and lateral wall of the nose to place it in the inferior meatus
- Dacryocystorhinostomy (DCR)
- Can be performed externally or endonasally
- Lacrimal sac fossa (DCR ostium) corresponds to anterior tip/axilla of middle turbinate
- About 1/3rd of lacrimal sac lies superior to axilla of middle turbinate
- Agger nasi cell often intervenes between lacrimal sac and nasal cavity and must be removed during DCR.
References and additional resources
- Annino DJ Jr, Goguen LA. Silent sinus syndrome. Curr Opin Otolaryngol Head Neck Surg. 2008; 16:22-25.
- Bilyk JR. Periocular infection. Curr Opin Ophthalmol. 2007; 18:414-423.
- Burm JS, Chung CH, Oh SJ. Pure orbital blow out fracture: New concepts and importance of medial orbital blowout. Plast Reconstr Surg. 1999; 103:1839-1849.
- Burnstine MA. Clinical recommendations for repair of isolated orbital floof fractures. Ophthalmology. 2002; 109:1207-1213.
- Chang WJ, Tse DT, Bressler KL, et al. Diagnosis and management of allergic fungal sinusitis with orbital involvement. Ophthal Plast Reconstr Surg 2000;16:72.
- Cho RI, Davies BW. Combined orbital floor and medial wall fractures with involvement of the inferomedial strut: repair technique and case series. Craniomaxillofac Trauma Reconstr 2013;6:161-9.
- Converse JM, Smith B. On the treatment of blowout fractures of the orbit. Plast Recons Surg 1984;54:81.
- Dailey RA, Cohen JI. Surgical repair of the silent sinus syndrome. Ophthal Plast Reconstr Surg 1995;11:261.
- Dalgorf DM, Harvey RJ. Sinonasal anatomy and function. Am J Rhinol Allergy 2013;27:S3-6.
- Doonquah L, Brown P, Mullings W. Management of frontal sinus fractures. Oral Maxillofacial Surg Clin N Am 2012;24:265-74.
- Djupesland PG, Chatkin JM, Qian W, Haight JSJ. Nitric oxide in the nasal airway: a new dimension in otorhinolaryngology. Am J Otolaryngol 2001;22:19-32.
- Frazell EL, Lewis JS. Cancer of the nasal cavity and accessory sinuses: a report of 116 patients. Cancer 1963;16:1293.
- Garcia GH, Harris GJ. Criteria for nonsurgical management of subperiosteal abscess of the orbit: analysis of outcomes 1988-1998. Ophthalmology 2000;107:1454-8.
- Graney DO, Rice DH. Chapter 55: Anatomy. In Cummings CW, Frederickson JM, Harker LA, et al, ed. Otolaryngology – Head and Neck Surgery, 3rd Ed. St. Louis: Mosby-Year Book, 1998.
- Harman LE, Margo CE. Wegener’s granulomatosis. Surv Ophthalmol 1996;42:458-80.
- Kazim M, Calsina M. Surgical decompression for thyroid eye disease. In: Black EH, et al, ed. Smith and Nesi’s Ophthalmic Plastic and Reconstructive Surgery, 3rd Ed. New York: Springer, 2012.
- Keir J. Why do we have paranasal sinuses? J Laryngol Otology 2009;123:4-8.
- Lentworth LN, Krohel GB, et al. Sinus tumor invading the orbit. Ophthalmology 1984;91:209.
- Levin LA, Avery R, Shore JW, et al. The spectrum of orbital aspergillosis: a clinicopathological review. Surv Ophthalmol 1996;41:142-54.
- Ogle OE, Weinstock RJ, Friedman E. Surgical anatomy of the nasal cavity and paranasal sinuses. Oral Maxillofacial Surg Clin N Am 2012;24:155-66.
- Parker NP, Pearlman AN, Conley DB, et al. The dilemma of midline destructive lesions: a case series and diagnostic review. Am J Otolaryngol 2010;31:104-9.
- Schwarz JN, Donnelly EH, Klintworth GK. Ocular and orbital phycomycosis. Surv Ophthalmol 1977;22:3-28.
- Sisson GA, Johnson NE, Amiri CS. Cancer of the maxillary sinus; clinical classification and management. Ann Otolaryngol 1963;72:1050.
- Van Cauwenberge P, Sys L, De Belder T, Watelet JB. Anatomy and physiology of the nose and paranasal sinuses. Immunol Allergy Clin N Am 2004;24:1-17.
- Warner N, Eggenberger E. Traumatic optic neuropathy: a review of the current literature. Curr Opin Ophthalmol 2010;21:459-62.
- Watters EC, et al. Acute orbital cellulitis. Arch Ophthalmol 1976;4:785.
- Weber AL, Mikulis DK. Inflammatory disorders of the paraorbital sinuses and their complications. Radiol Clin North Am 1987;25:615.
- Weitzel EK, Cho RI. Endoscopic Orbital and Lacrimal Surgery. In: Johnson JT, Rosen CA, ed. Bailey’s Head and Neck Surgery – Otolaryngology, 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2014.
