Eyelid Pediatric Ptosis
Updated July 2024
Congenital ptosis is present at birth, though it may not be appreciated until a few weeks to months of age. This outline will include both congenital and acquired pediatric ptosis.
Establishing the diagnosis
Etiology
- Congenital myogenic ptosis (isolated congenital ptosis)
- Unknown cause — characterized by a maldevelopment of the levator muscle and/or aponeurosis
- The levator muscle is variably replaced with fibrous or fatty tissue
- Congenital neurogenic ptosis — due to defects during embryonic development or perinatal trauma
- Congenital cranial nerve (CN) III palsy — might be due to birth trauma or ischemic insult to brain
- Usually unilateral
- Variable limitation of elevation, depression and adduction of the globe
- Dilated pupil
- Can have aberrant reinnervation
- Frequently causes amblyopia
- Congenital Horner syndrome
- Interruption of the sympathetic pathway
- Birth trauma or neck trauma
- Neuroblastoma
- 1 to 2 mm of upper eyelid ptosis
- 1 mm of reverse (inverse) ptosis of the lower eyelid
- Miosis with dilation lag
- Anhidrosis if interruption proximal to carotid bifurcation
- Iris heterochromia, with ipsilateral iris lighter in color
- Marcus Gunn jaw-winking syndrome
- Aberrant connections between motor division of trigeminal nerve and oculomotor nerve
- Unilateral (or rarely bilateral) upper eyelid ptosis with elevation of the ptotic lid with opening of the jaw, or movement of the jaw to the contralateral side
- Variably decreased levator function
- Can have decreased superior rectus function
- Genetics
- Blepharophimosis syndrome (FOXL2).
- PTOS1 = hereditary congenital ptosis gene 1 — a possible balanced translocation between 1p34 32 and 8q21
- PTOS 2 = a dominant x linked rearrangement
- ZXFH4 = zinc finger homeobox 4 — encodes a protein involved in neural and muscle differentiation, maps to 8q13 q21.
- X-linked (Xq24)
- Acquired pediatric neurogenic ptosis
- Cranial nerve (CN) III palsy — associated with trauma or intracranial mass
- Horner syndrome — associated with head, neck, and chest trauma or neoplasms
- Ophthalmoplegic migraine
- Aponeurotic ptosis — might be caused by eyelid trauma, birth trauma from forceps or suction delivery, or history of contact lens wear
- Patients can have a high lid crease due to a disinsertion of the levator aponeurosis
- Levator function is often normal
- Traumatic ptosis — injury to the elevators of the eyelid (levator aponeurosis and/or Müller’s muscle)
- Can improve over time with healing or mass effect from edema or hemorrhage (mechanical ptosis)
- Mechanical ptosis — mass effect from an eyelid or orbital mass (eg, chalazion, preseptal or orbital cellulitis, neurofibroma, infantile hemangioma)
Epidemiology
- Congenital myogenic ptosis affects males and females equally
- Usually not inherited in the isolated form
- The most common form of pediatric ptosis
- The incidence of pediatric ptosis was measured over a 40-year period (1965–2004) in Olmsted County, Minnesota (Ophthalmology 2011;118:1180).
- 107 patients younger than 19 years of age were diagnosed with pediatric ptosis
- Incidence 7.9 per 100,000, prevalence 1 in 842 births.
- 75% were simple congenital ptosis
- 55% male, 45% female
History
- Congenital myogenic ptosis is typically constant after birth
- Onset — when was ptosis first noticed — typically at birth or days to weeks after birth
- Laterality — unilateral or bilateral
- Duration — constant, intermittent, progressive
- Associated movements — eyelid jumping when chewing, babbling, or yawning
- Birth history — vaginal versus C section, suction or forceps in delivery
- Family history — ptosis or blepharophimosis, strabismus, syndromic disease such as congenital fibrosis, double elevator palsy, neurofibromatosis
Clinical features
- External photographs — family photos of the child are helpful to confirm the onset and severity
- Photographs in the office are often best taken before contact with a young child — try a fixation object, and when the patient is not smiling
- Vision — screen for deprivation amblyopia due to ptosis, or from induced astigmatism
- Penlight test for light aversion is the simplest vision test
- Object fixation testing with a flashing object or toy to assess tracking can be accomplished within 4–8 weeks of age
- Children over 3 months can be tested for gaze preference with Teller visual acuity cards
- Patients 18 months and older can be tested by matching shapes, HOTV, and Snellen charts
- Pupil examination — anisometropia might indicate Horner’s syndrome, or 3rd nerve palsy
- Ocular alignment — incomitant deviation might indicate 3rd nerve palsy, double elevator palsy, orbital fibrosis or myasthenia gravis
- Comitant deviation would indicate possible strabismic amblyopia
- Medial canthal displacement — telecanthus, hypertelorism and epicanthal folds
- Margin reflex distance 1 (MRD1) in mm = eyelid height as measured in mm from the center of the cornea to the central eyelid margin
- Margin reflex distance 2 (MRD2) in mm = measured from center of the cornea to the lower eyelid margin, can be reduced in Horner’s syndrome
- Levator function — without brow use — examiner’s thumb splints the eyebrow when measuring eyelid excursion from up to downgaze
- Characterization of levator function
- Excellent (≥ 13 mm)
- Good (8–12 mm)
- Fair (5–7 mm)
- Poor (≤ 4 mm)
- Bell’s phenomenon — involuntary upgaze with eyelid closure — absence increases the risk of corneal exposure after ptosis repair
- Corneal sensation can be tested with a piece of tissue or cotton
- Frontalis function — ability to raise the eyebrows should be noted
- Orbicularis function — orbicularis weakness might indicate congenital 7th nerve palsy
- Head position — chin up or head tilt
- Cycloplegic refraction — corneal curvature can be altered by ptosis and eyelid surgery — can be performed on initial examination, and 3–6 months after ptosis surgery
- Phenylephrine test — even patients with severe ptosis and poor levator function can have a good response to this test
- Phenylephrine response can be documented with photographs
- MRD1 before and 5 minutes after 2.5% phenylephrine administration
- Test more ptotic eye first
- Contralateral ptosis after phenylephrine drop might be due to Hering’s law
- The use of 10% phenylephrine should be avoided in children
- Visual field — might be necessary for insurance coverage in older children
- An additional clinical pearl — often newborns are seen in the office sleeping. Even after arousal infants can remain somnolent. A great examination tool is to elicit the eye-popping reflex. This is where a newborn will open his or her eyes when the room lights are dimmed or turned off.
Testing and evaluation for establishing the diagnosis
- Imaging
- Unnecessary in cases of isolated congenital ptosis
- MRI of the orbits for suspected cases of mechanical ptosis (eg, due to large orbital hemangioma)
- MRI from brain to thorax for suspected Horner’s
- Consider orbital imaging if proptosis is present
- Consider additional imaging if there is a concern for neuroblastoma
- Consider work-up for myasthenia gravis (MG)
Risk factors
- Congenital myogenic ptosis usually occurs as an isolated finding
- Systemic diseases associated with ptosis
- Saethre-Chotzen syndrome
- Noonan syndrome
- Trisomy 21
- Mowat-Wilson syndrome
- Polycystic kidney disease
- Neurofibromatosis Type 1
- Costello syndrome
- Joubert syndrome
- Cornelia de Lange syndrome
Differential diagnosis
- Pseudoptosis
- Contralateral eyelid retraction
- Facial asymmetry (eg, plagiocephaly)
- Contralateral proptosis
- Enophthalmos
- Eyelid inflammation (eg, conjunctivitis)
- Microphthalmos, anophthalmos or nanophthalmos
- Phthisis bulbi
- Anisometropia (with smaller eye being more hyperopic or less myopic)
- Vertical strabismus
Patient Management
Natural history
- Ptosis can improve or resolve with time in certain circumstances
- Mechanical ptosis due to infections or excisable mass
- Traumatic ptosis including birth trauma can resolve
- A small percentage of pediatric ocular myasthenia will spontaneously resolve
- Adequate follow-up to monitor vision is important to prevent deprivation amblyopia
- Consider prophylactic contralateral eyelid patching or cycloplegia during observation period
- Ptosis that occludes the central visual axis or with documented asymmetric vision is an indication for patching or cycloplegia
- Consider amblyopia therapy even if surgery is pending for cases of severe ptosis (MRD1 ≤ 1mm)
- The incidence of amblyopia in congenital ptosis is approximately 18% (reports have ranged from 11% to 25%) (J Pediatr 2014; 165:820)
- Correct refractive error — ptosis can induce astigmatism with mechanical corneal modeling
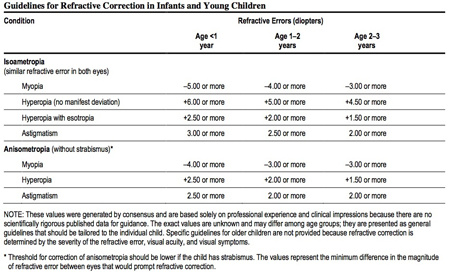
Table 1. Guideline for refractive correction for infants and young children that should be treated with glasses.
Medical therapy options
- No medical treatment for congenital myogenic ptosis
Radiation therapy options
- None
Surgery options
- Indications
- The presence or risk of amblyopia due to covering or encroachment of the central visual axis
- Psychosocial: Any eyelid asymmetry should be seen as a potential cause for body dysmorphism. It is important to take into account both the patient’s and the parent’s desires to achieve eyelid symmetry
- Timing
- If good vision is documented there is no urgency for ptosis repair
- After 6 months of age elective surgery is considered to be of a lower risk for general anesthesia
- Severe ptosis that covers the central visual axis such as with congenital 3rd nerve palsy might require earlier repair
- Mechanical ptosis (eg, large infantile hemangioma) that has not responded well to medical management might require removal of the mass
- If child has a planned general anesthesia for another problem ptosis repair can be arranged at same anesthesia
- Goal of surgery
- Functional success
- Eyelid height is elevated to clear the central visual axis (Usually an MRD1 of ≥2mm)
- Chin up position is resolved or improved due to better eyelid height
- Excessive brow use can be resolved in younger children, but not always
- Cosmetic success
- A functional success with good eyelid symmetry and contour as compared to the fellow eye. The eyelid height should have an MRD1 of ≥ 3mm in most patients (Figure 1)
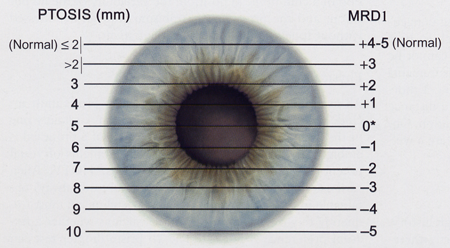
Figure 1. The margin reflex distance 1 (MRD1) as measured in millimeters from the center of the cornea (right column of numbers). This is compared to an estimated amount of ptosis as measured from the superior corneal limbus (left column of numbers). It is best when describing ptosis to make MRD1 measurements.
- Choice of procedure
- Müllerectomy (Putterman’s technique) with or without a tarsectomy
- Good response to 2.5% Phenylephrine is a good predictor of surgical success
- Indication might be patients at high risk of exposure keratopathy with poor or missing Bell’s phenomenon
- Anterior levator resection
- Fair to excellent levator function (≥ 5 mm)
- Frontalis sling with synthetic or banked sling material
- Poor levator function (≤4mm)
- Age below 4 years (insufficient leg length for autogenous fascia lata graft)
- Potential need for removal (eg, 3rd nerve palsy with risk of cornea exposure)
- Frontalis sling with autogenous fascia lata
- Poor levator function (≤ 4mm)
- Age above 4 years (adequate leg length for autogenous fascia lata harvesting — ideal graft is ≥12cm)
- Frontalis muscle advancement
- Poor levator function (≤ 4mm)
- Frontalis muscle is well developed by 2 years of age (Goldey, OPRS, 2000)
- Can safely be performed in patients under age 2 (Medel, Orbit 2014)
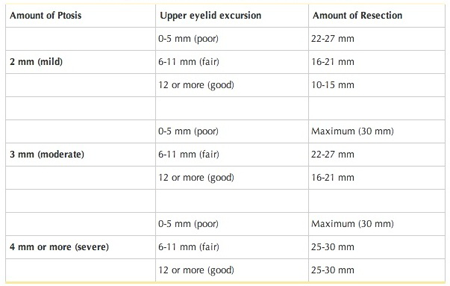
- Table 2 depicts Beard’s selection of ptosis repair based on levator function.
Table 2. Selection of ptosis repair based on levator function.
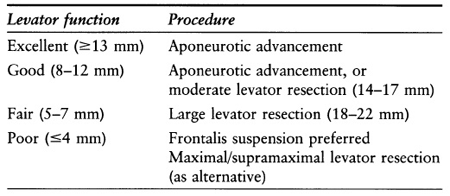
- Types of Müller’s muscle surgery
- Modified Fasanella Servat
- Ideal procedure for mild ptosis with a good response to 2.5% phenylephrine
- Surgical outcome usually will be close to the result of a good phenylephrine response
- Two matching hemostats are used (or a Putterman clamp) over an everted eyelid to crush the tarsus, conjunctival and Müller’s muscle at about 4–5mm from the eyelid margin
- This is then excised and anastomosed with a gut or nonabsorbable suture
- If a Nylon or Prolene suture is used it should be removed in 5–7 days
- Putterman procedure — Müller’s muscle conjunctival resection (MMCR)
- Also ideal procedure for mild ptosis with a good phenylephrine response
- The conjunctival and Müller’s muscle are measured with calipers for a desired amount of resection and held on traction with a suture or skin hook
- A Putterman clamp is used to isolate this complex for ease of resection and suturing
- Variants of Fasanella Servat
- Can remove 9mm of Müller’s muscle and 1mm of tarsus for every under corrected 1mm on phenylephrine testing (OPRS 2002; 18:426)
- Can remove 2 mm of tarsus for each mm of desired eyelid elevation (OPRS 2013; 29:30)
- Can remove 2mm of Müller’s muscle for each 1mm of under corrected ptosis on phenylephrine testing
- For patients at higher risk of exposure keratopathy, for developmental or behavioral reasons, ptosis procedures such as frontalis slings and levator resection should be avoided to minimize postoperative lagophthalmos
- Modified FS can be used in these cases emphasizing functional outcome while sacrificing cosmetic outcome for the benefit of the patient
- Anterior levator resection (Figures 2 and 3)
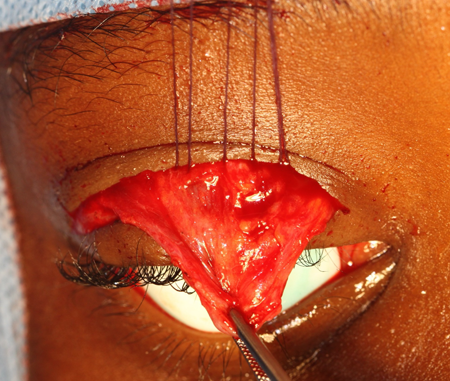
Figure 2. Levator resection. Intraoperative photo of a levator muscle dissection with three 5-0 Vicryl sutures passed from the mid-tarsus to the levator aponeurosis (anterior to Whitnal ligament).
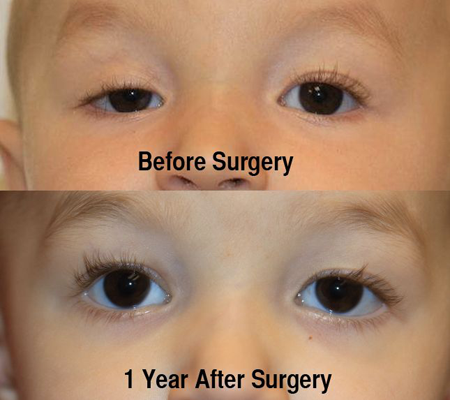
Figure 3. A. Preoperative photo of a patient with right-sided ptosis with good levator function. B. Postoperative photo 1 year after a right anterior levator resection.
- Ideal for a patient with a moderate to excellent levator function (6mm and above) and poor response to 2.5% phenylephrine.
- Nomogram 1 (Table 3) created by Berke in 1961 targets intraoperative eyelid height based on preoperative levator function.
Table 3. Amount of levator surgery for congenital, myogenic ptosis based on levator function and intraoperative eyelid level (under general anesthesia).

- Nomogram 2 (Table 4) created by Beard in in 1976 correlates amount of levator resection with amount of ptosis.
Table 4. Estimation of levator resection (Beard 1976).
- An eyelid skin crease incision is made and the levator muscle is advanced to the tarsus with an absorbable suture (5- or 6-0 Vicryl)
- Excess aponeurosis is excised when the desired intraoperative height is achieved
- Frontalis Suspension (Sling):
- Indications
- Congenital ptosis with poor levator function (usually less than 4 mm of excursion)
- Neurogenic ptosis with poor levator function
- Traumatic ptosis with poor levator function
- Mechanical ptosis where the mass effect on the eyelid is still amblyogenic.
- Contraindications
- If good levator function is present and alternative should be considered first
- Significant keratopathy
- Missing corneal sensation
- Complete facial nerve palsy with absent frontalis function
- Caution if poor motility or absent Bells phenomenon
- Sling materials
- Synthetic: Silicone, expanded polytetrafluoroethylene (ePTFE (Gortex)), Nylon monofilament (Supramid), or Ptose-up
- Banked fascia (Tutoplast)
- Autologous fascia lata
- Sling configurations
- Triangle — 1 brow incision
- Rhomboid — 2 brow incisions
- Pentagon — 3 brow incisions
- Double Triangle — 2 or 3 brow incisions (Figure 4)
- Double Rhomboid — 3 brow incisions
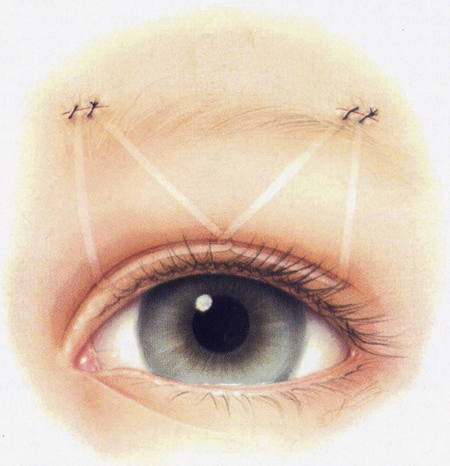
Figure 4. Frontalis suspension: double-triangle technique. In this illustration, the material is locked (crossed) in the center of the eyelid (optional).
- Choice of sling material
- All nonautologous materials carry a higher risk for rejection and infection
- Silicone slings are very well tolerated
- Figure 5 depicts a silicone sling (Seiff set, FCI Ophthalmics) placed in a rhomboid configuration with 2 brow incisions
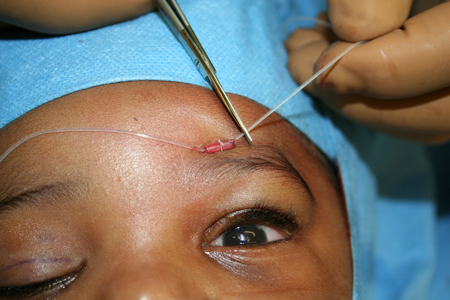
Figure 5A. Intraoperative photo of frontalis suspension with silicone sling placed in a rhomboid fashion. The silicone material is being tightened through a sleeve until the desired eyelid height is reached.
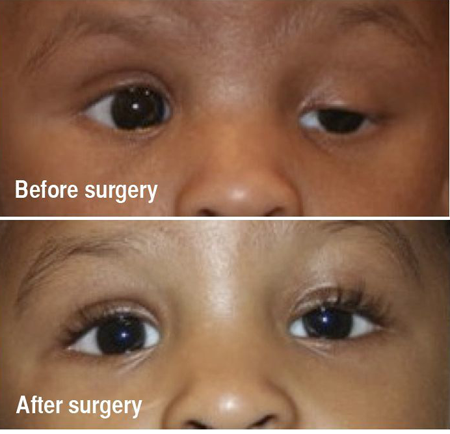
Figure 5B. Top: Preoperative showing severe left ptosis with poor levator function and a missing eyelid crease. Bottom: Postoperative 1 year after a left sided silicone frontalis suspension.
- Autologous fascia lata is very well tolerated and can be harvested when a patient is tall enough (Ideally over the age of 4–5 years).
- Frontalis Suspension (Muscle Suspension):
- Can be used for revision congenital ptosis (Dallalzadeh, J Craniofac Surg, 2021)
- Aims to link the frontalis muscle to the upper eyelid tarsus with bypassing the levator muscle
- Less risk of foreign body granuloma, late exposure and excision on the forehead
Describe other management considerations
- Levator extirpation
- Can be considered to remove a Marcus Gunn jaw wink. To be performed when a frontalis sling is placed
- Whitnall’s sling
- This is an alternative to frontalis sling surgery where only the aponeurosis is excised and Whitnall’s ligament and the underlying levator muscle is sutured directly to the tarsus (Arch Ophthalmol 1990; 108:1628)
- Temporary suture tarsorrhaphy
- In the setting of a maximal levator resection or frontalis sling surgery a temporary lateral suture tarsorrhaphy can be placed
- This can decrease the initial risk of postoperative corneal exposure
- Can be removed 2–7 days after surgery when orbicularis compliance (relaxation) is improved and lagophthalmos is improved
- Unilateral versus bilateral surgery
- Asymmetric preoperative ptosis is always a challenge
- Pediatric patients are unable to participate to assess intraoperative eyelid height
- In the setting of unilateral ptosis, contralateral ptosis might become uncovered due to Herring’s law (Figure 6)
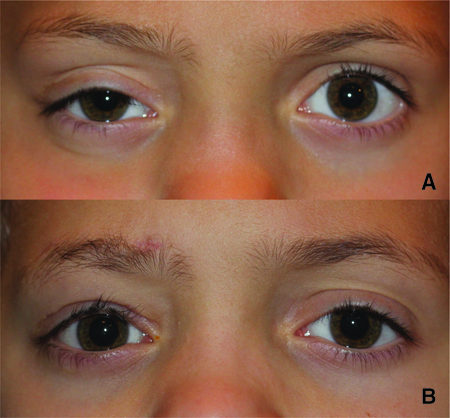
Figure 6. Right-sided ptosis with mild left upper eyelid retraction. B. Demonstration of Herring’s law. Postoperative photo 1 year after a right autogenous fascia lata frontalis suspension sling. The left upper eyelid is now lower in height.
- Bilateral symmetric ptosis repair where the same procedure is performed on both eyelids may allow for better symmetry, but may be unnecessary. This is in contrast to a per-eyelid approach where the choice of procedure is made independent of each eyelid (eg, right Fasanella for mild ptosis with a left sling for severe ptosis)
Preventing and managing treatment complications
- Infection
- Minimize risk with careful, standard, aseptic technique
- Treat with antibiotics
- Over or under correction
- Revise as needed
- Trial of massage for overcorrection, if not effective then recess
- Consider the possibility of sling material failure in sudden postoperative ptosis (eg, sling breakage or cheese-wiring)
- Wound dehiscence
- Repair wound
- Corneal exposure or ulcer
- Consider temporary tarsorrhaphy for sling and large levator resections
- Keep a low threshold to place a tarsorrhaphy in any case of a large postoperative corneal abrasion that does not heal within a day
- Poor contour
- Prevent with careful surgical technique
- Surgically revise as needed
- Upper eyelid entropion
- May need lash rotational sutures
- Lagophthalmos on downgaze
- Expected side effect with large levator advancement/resection or sling
- Lagophthalmos
- Expected side effect with large levator advancement/resection or sling
- Treat with lubricating drops or ointment
- Consider frequent postoperative visits to monitor corneal health
- Bleeding
- Careful surgical technique and hemostasis
- Open wounds if vision is threatened
- Observe if vision is not threatened
- Scar
- Careful wound closure
- Treat with massage, corticosteroids (topical or injected); surgical revision when needed
- Consider silicone gels to the brow incisions to help prevent scar hypertrophy after there is adequate skin epithelialization
- Wound granuloma
- Greater risk with synthetic materials.
- Some patients are at higher risk (eg, Ataxia-Telangiectasia)
- Lid crease asymmetry
- Prevent with careful planning of incision site
- Revise surgically as needed
- Conjunctival prolapse
- Avoid damage to the suspensory connections between the levator and the superior conjunctival fornix
- May resolve with patching
- Tissue may need to be repositioned and secured with full-thickness sutures passed through fornix
- Dry Eye
- Frequent application of ocular lubricants may be necessary.
- For patients with slings lubrication over night for severe lagophthalmos may be necessary.
- Suture abscess, infected or exposed sling material
- Antibiotics, topical or oral
- Removal of sling material
- Repair of sling exposure or extrusion
- Medical-surgical management of granuloma or suture abscess
Historical perspective
- Fasanella-Servat (FS) procedure described in 1961 (Arch Ophthalmol 1961; 65:493).
- Modified by Beard in 1970 (Am J Ophthalmol 1970; 69:850).
- Putterman introduced a clamp to replace the 2 curved hemostats used in the FS (Arch Ophthalmol 1972; 87:665).
- Frontalis muscle advancement was first introduced in 1901 by Fergus and re-introduced in 1982 by Song (Fergus, Br Med J 1901, Song, Clin Plast Surg, 1982)
References
- Fibrosis of extraocular muscles, congenital, 1; CFEOM1
- Pediatric Eye Evaluations PPP — 2012
- Anderson RL, Jordan DR, Dutton JJ. Whitnall’s sling for poor function ptosis. Arch Ophthalmol. 1990;108(11):1628 1632.
- Beard, C. Blepharoptosis repair by modified Fasanella-Servat operation. Am J Ophthalmol. 1970;69(5):850 857.
- Beard C. Newer ptosis procedures. In: Ptosis, 2nd ed. St. Louis: Mosby; 1976:189 212.
- Berke RN. Resection of the levator muscle through the external approach for congenital ptosis. Trans Pac Coast Otoophthalmol Soc Annul Meet. 1964;45:207 214.
- Carter SR, Meecham WJ, Seiff SR. Silicone frontalis slings for the correction of blepharoptosis: indications and efficacy. Ophthalmology. 1996;103:623 630.
- Cook-Sather SD, Schreiner MS. Pediatric anesthesia techniques. In: Katowitz JA, ed. Pediatric Oculoplastic Surgery. New York: Springer-Verlag. 2002:61 82.
- Cruz AAV, Akaishi APMS. Frontalis-orbicularis muscle advancementfor correction of upper eyelid ptosis: a systematic literature review. Ophthalmic Plast Reconstr Surg2018;34:510–51.
- Dallalzadeh LO, Park KS, Korn BS, Kikkawa DO, Liu CY. Minimal Dissection Direct Frontalis Muscle Advancement Flap for Congenital Ptosis Repair. J Craniofac Surg. 2021 Oct 1;32(7):2358-2361.
- Fasanella RM, Servat J. Levator resection for minimal ptosis: another simplified operation. Arch Ophthalmol. 1961;65:493 496.
- Fergus F. An easy operation for congenital ptosis.Br Med J1901;1:76218.
- Goldey SH, Baylis HI, Goldberg RA, Shorr N. Frontalis muscle flap advancement for correction of blepharoptosis. Ophthal Plast Reconstr Surg 2000;16:83–93.
- Griepentrog GJ1, Diehl NN, Mohney BG. Incidence and demographics of childhood ptosis. Ophthalmology. 2011;118(6):1180 1183.
- Heher KL, Katowitz JA. Pediatric Ptosis. Katowitz JA; Pediatric Oculoplastic Surgery. New York: Springer-Verlag; 2002:262 280.
- Nakashima M, Nakano M, Hirano A, et al. Genome-wide linkage analysis and mutation analysis of hereditary congenital blepharoptosis in a Japanese family. J Hum Genet. 2008;53(1):34 41.
- Perry JD, Kadakia A, Foster JA. A new algorithm for ptosis repair using conjunctival Müllerectomy with or without tarsectomy. Ophthal Plast Reconstr Surg. 2002;18(6):426 429.
- Putterman, AM. A clamp for strengthening Müller’s muscle in the treatment of ptosis. Modification, theory, and clamp for the Fasanella-Servat ptosis operation. Arch Ophthalmol. 1972;87(6):665 667.
- Samimi DB, Erb MH, Land, CJ, Dresner SC. Modified Fasanella-Servat Procedure: Description and Quantified Analysis. Ophthal Plast Reconstr Surg. 2013;29:30 34.
- Smit D. Pharmacological testing in Horner’s syndrome – a new paradigm. South African Medical Journal. 2010;100(11):738 740.
- Song R, Song Y. Treatment of blepharoptosis. Direct transplantation ofthe frontalis muscle to the upper eyelid.Clin Plast Surg1982;9:45–4819.
- Stein A, Kelly JP, Weiss AH. Congenital eyelid ptosis: onset and prevalence of amblyopia, associations with systemic disorders, and treatment outcomes. J Pediatr. 2014;165(4):820 824.
