Orbital Rhabdomyosarcoma
Updated May 2025
Proptosis in childhood can represent a vision- or life-threatening condition such as infection or malignant neoplasm and requires a prompt and thorough evaluation. Rhabdomyosarcoma is the most common malignant orbital tumor of childhood, and affected patients often first present to the ophthalmologist. The rapid diagnosis and appropriate treatment of this cancer can be life saving.
Establishing the diagnosis
Etiology
- Tumor cells originate from primitive pleuripotential mesenchymal cells that possess the ability to differentiate into striated muscle (Shields, Surv Ophthalmol 2003).
- No known specific genes suggesting direct hereditary transmission
- Associated with other diseases: neurofibromatosis, and Li-Fraumeni syndrome, which is associated with germline mutation p53 suppressor gene (Li, J Nat Cancer Inst, 1969)
- Can occur in head and neck region, retroperitoneum, perineum, as well as the orbit, conjunctiva, eyelid, and anterior uvea (Shields, Ophthalmology, 2001)
- Represents 5% of all childhood cancers, with about 350 cases diagnosed in the US per year, of which about 10% are orbital tumors (Maurer, Cancer, 1988 and 1993)
- Can be primary, secondary (paranasal sinuses or nasopharynx), or metastatic
- Orbital tumors have a high cure rate and carry the best prognosis (Raney, Natl Cancer Inst Monogr, 1981).
Epidemiology
- Most common primary orbital malignancy of childhood (Friedmann, N Eng J Med, 2004)
- Onset: mean age 8 years typical, broad range, from birth to 78 years reported (Shields, Surv Ophthalmol, 2003)
- Slight predilection for males
- Less commonly can present in conjunctiva, eyelid, iris, or ciliary body
- Invariably unilateral
History
- Rapid onset of unilateral proptosis
- Absence of fever, chills, malaise
- Eyelid swelling, conjunctival congestion
- Decreased ocular motility
- Pain and decreased vision are uncommon
Clinical features
- Proptosis, often downward displacement of globe (Figure 1, Figure 2)
- Palpable mass
- Ptosis
- Strabismus
- Lid edema and erythema
- Conjunctival chemosis
- Decreased ocular motility
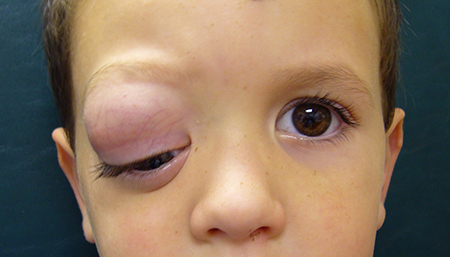
Figure 1. A 3-year-old boy presents with painless proptosis of his right eye for 3 months. He has a large superior orbital mass pushing his right eye downward.
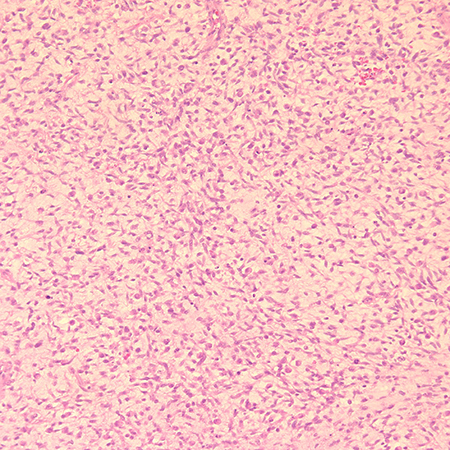
Figure 2. Biopsy shows a tumor with round to oval hyperchromatic nuclei with abundant eosinophilic cytoplasm. Mitotic figures are seen. Diagnosis is embryonal rhabdomyosarcoma. Hematoxylin and eosin, magnification x 20.
Testing
- Computed tomography (CT) scan (Figure 3)
- Solid mass with moderate enhancement
- Isodense to extraocular muscles
- Rare to have bony destruction early in course of disease, but bone changes can be seen in up to 40% of patients (Sohaib, Clinical Radiology, 1998)
- Rapid growth of lesion can indent the globe
- Predilection for superonasal orbit with most common cell type (embryonal) (Sohaib, Clinical Radiology, 1998)
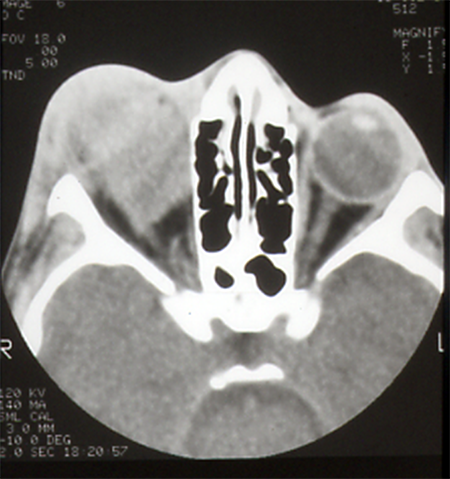
Figure 3. CT scan of 9-year-old boy reveals a superior diffuse orbital mass isointense to extraocular muscles. Pathology revealed an embryonal rhabdomyosarcoma.
- Magnetic resonance imaging (MRI)
- Relative to extraocular muscles, the mass is isointense on T-1-weighted image and slightly hyperintense on T-2-weighted image (Sohaib, Clinical Radiology, 1998)
- Densely and homogeneously enhances after administration of gadolinium (Friedmann, N Eng J Med 2004)
- Early management
- Thorough history and physical examination
- Prompt biopsy and/or debulking of lesion if it can be safely debulked without damaging adjacent structures (Shields, Ophthalmology, 2001)
- Pediatric oncology and radiation therapy consultation as soon as possible
- Diagnosis
- Pathologic confirmation
- Four subtypes: embryonal, pleomorphic, alveolar, and botryoid (Newton, J Clin Oncol, 1998)
- Embryonal most common, accounting for about 80%. Displays round to spindle-shaped elongated cells with eosinophilic cytoplasm, hyperchromatic nuclei, sometimes cross-striations within cytoplasm.
- Alveolar shows loosely arranged cells resemble lung alveoli and has the worst prognosis. This subtype demonstrates frequent cytogenetic abnormalities (Friedmann, N Eng J Med 2004).
- Botryoid appears grapelike, does not occur primarily in the orbit, but can secondarily invade from conjunctiva or paranasal sinuses, and has the best prognosis.
- Pleomorphic
- Immunohistochemical staining includes positivity for desmin, antimuscle-specific actin (MSA), myoglobin, myosin (Figure 4, Figure 5) (Friedmann, N Eng J Med 2004; Scupham, Arch Pathol Lab Med 1986; Parham, Cancer 1991).
- Metastatic workup
- Chest x-ray
- Bone marrow biopsy
- Lumbar puncture
- Lung parenchyma is the most common site of metastasis, followed by bone marrow, bone, and regional lymph nodes
- Staging of Tumor (Crist, J Clin Oncol 1995)
- Group I: localized disease completely resected
- Group II: regional disease, grossly resected
- Group III: gross residual disease after surgery
- Group IV: metastatic disease
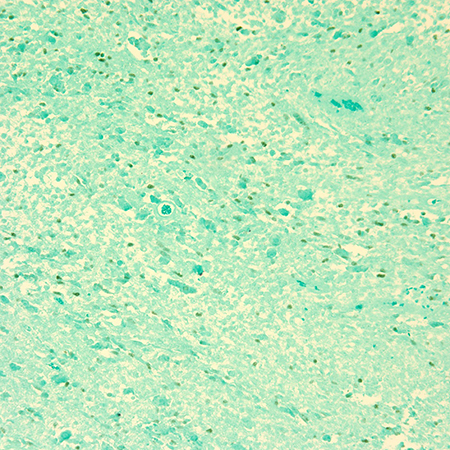
Figure 4. Immunohistochemical stain with Myogenin stains nuclei green in this case of embryonal rhabdomyosarcoma. Magnification x 20.
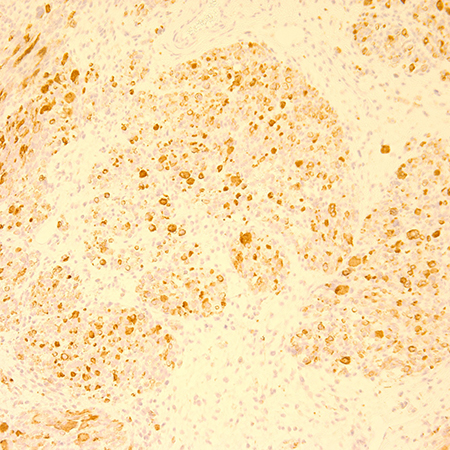
Figure 5. Immunohistochemical stain with Desmin consistent with diagnosis of rhabdomyosarcoma. Magnification x 20.
Differential diagnosis
- Orbital cellulitis
- More inflammatory signs
- Fever, malaise
- Increased white blood cell count with left shift
- Pain more common
- Concomitant sinusitis common
- Idiopathic orbital inflammation
- More inflammatory signs
- Pain common
- Can be diffuse or localized to an extraocular muscle or lacrimal gland
- Neuroblastoma
- Increased vanillylmandelic acid (VMA)
- Orbit involvement often bilateral
- Predilection for lateral orbit with bony destruction
- Eyelid ecchymosis more common
- Lymphangioma with abrupt hemorrhage (chocolate cyst)
- Ruptured dermoid cyst
- Langerhan Cell Histiocytosis (Eosinophilic granuloma, Letterer-Siwe, Hand-Schüller-Christian,
- Juvenile xanthogranuloma
- Leukemic infiltrate (granulocytic sarcoma) or lymphoma
- Ewing’s sarcoma
- Fibrosarcoma
Patient management: treatment and follow-up
Medical therapy
- Chemotherapy
- Traditionally the main agents have included vincristine, actinomycin, and cyclophosphamide (VAC).
- Newer agents include etoposide/ifosfamide, which are especially helpful in high risk or refractory cases, and topotecan and irinotecan (Crist, J Clin Oncol, 1995 and 2001; Ruymann, Cancer Invest, 2000; Arndt, Pediatr Blood Cancer, 2008). Recent studies support the use of maintenance chemotherapy with vinorelbine and low-dose cyclophosphamide, showing improved progression-free and overall survival in intermediate-risk rhabdomyosarcoma patients (Pan 2024).
- The RMS13 trial reported a 5-year overall survival of 75.1% and event-free survival of 67.5% with post-induction maintenance therapy using cyclophosphamide, bevacizumab, and sorafenib (Pan 2024).
- Radiation therapy (RT)
- External beam
- Fractionated doses with total amount of radiation about 4,500–5,000 cGy
- Significant side effects including developmental delay and second tumor
- Some protocols in low-risk patients use chemotherapy alone without RT (Raney, J Pediatr Hematol Oncol, 2001), (Crist, J Clin Oncol, 2001)
- Proton beam
- Superior to external beam (photon irradiation) and has excellent tumor coverage with less collateral damage.
- The physical properties of photons result in rapid loss of energy at the end of the range of the beam.
- Limits the dose to brain, pituitary, hypothalamus, temporal lobes, and ipsilateral and contralateral orbital structures, resulting in fewer side effects including developmental delay and secondary tumor, however, available in only a few centers (Yock, J Radiat Oncol Biol Phys, 2005; Friedmann, N Eng J Med 2004; Hug, Int J Radiat Oncol Biol Phys, 2000).
- Space-making particle therapy, involving surgical placement of a bioabsorbable spacer followed by proton beam therapy, has been utilized to protect adjacent organs and preserve function (Tokuda 2024).
Surgery
- After surgical biopsy and metastatic workup to stage the disease, the mainstay of treatment is nonsurgical, a combination of chemotherapy and radiation therapy (Karcioglu, Cancer Control, 2004).
- Under certain treatment protocols, surgical debulking of lesion if no risk of damage to surrounding structures
- In patients refractory to treatment with radiation and chemotherapy, exenteration is used. Survival rate is up to 71% at 3 years if tumor confined to orbit and combined with chemotherapy (Mannor, Ophthalmology, 1997).
Other considerations
- Combination of chemotherapy, radiation therapy and sometimes surgical debulking are used fairly successfully in the management of this disease.
- Prognosis is excellent if tumor treated appropriately while still confined to the orbit, and survival rate 93% at 5 years (Wharam,Ophthalmology, 1987).
- Embryonal cell type in the orbit has best prognosis with 5-year survival rate of 94% (Kodet, Med Pediatr Oncol, 1997).
- Alveolar cell type has worst prognosis with survival rate of 74% (Kodet, Med Pediatr Oncol, 1997).
- Infants less than 1 year of age have similar survival curve to older children (Ragab, Cancer, 1986).
Preventing and managing treatment complications
- Chemotherapeutic side effects
- Depends on agents used
- Bleeding, vision loss after biopsy
- Radiation side effects
- Developmental delay
- Second malignant tumor: osteosarcoma (Heyn ,J Clin Oncol, 1993)
- Radiation retinopathy
- Radiation optic neuropathy
- Radiation keratopathy
- Cataract (very common)
- Orbital hypoplasia with facial asymmetry
Disease-related complications
- Radiation keratopathy with severe dry eye
- Radiation induced cataract in up to 90%.
- Radiation optic neuropathy with visual loss
- Eyebrow loss due to radiation
- Death due to metastatic disease
- Statural growth retardation
- Metastasis, usually to regional lymph nodes, lungs or bone
- Intracranial extension
Research
The Intergoup Rhabdomyosarcoma Study (IRS) Group was established in 1972 by 3 pediatric cooperative cancer study groups. There have so far been 5 major trials, I–V. Survival after treatment of rhabdomyosarcoma at all sites has improved from 25% in 1970 to 70% in 1991 due to their work (Crist, J Clin Oncol, 1990; Maurer, Cancer, 1988; Wharam, Ophthalmology, 1987). The IRS found that favorable prognostic factors include
- Undetectable distant metastases at diagnosis
- Primary sites in the orbit and nonparameningeal head/neck and genitourinary nonbladder/prostate regions
- Grossly complete surgical removal of localized tumor at the time of diagnosis
- Embryonal/botryoid histology
- Tumor size < or = 5 cm
- Age younger than 10 years at diagnosis
Protocol V studied reducing exposure to cyclophosphamide and radiation therapy (RT) in patients at low risk while adding new, active agents such as topotecan or irinotecan to the standard therapy of vincristine, actinomycin D, and cyclophosphamide (VAC) plus XRT for patients with unfavorable histology or advanced disease. Bone marrow/stem cell reconstitution has not been included because this strategy has thus far failed to improve survival rates of patients with metastases at diagnosis (Raney, J Pediatr Hematol Oncol 2001).
A study conducted at the Amsterdam University Medical Centers analyzed 39 pediatric patients with primary orbital RMS treated between 1995 and 2016. The median follow-up period was 9.4 years. The 10-year overall survival rate was 95%, with an event-free survival rate of 63%. Local recurrences were observed in 14 patients, with most occurring within the first year post-treatment. Recurrences were more common in cases with intraconal and apical tumor locations. Notably, the study highlighted the importance of achieving local tumor control while preserving orbital function and aesthetics (Khatib 2025).
Research has shown that approximately one-third of patients may have residual orbital masses on imaging after completing multimodal treatment, including chemotherapy and radiotherapy. In some cases, these residual masses resolve spontaneously over time, while others may require surgical intervention. The presence of residual masses underscores the need for careful post-treatment monitoring and individualized management strategies (Sobel 2022).
Patient instructions
- Emphasize that this is a serious, life-threatening condition that needs to be managed urgently.
- Multidisciplinary treatment includes collaboration with ophthalmology, pediatric oncology, and radiation therapy.
- Frequent ocular lubrication during and after radiation therapy, possible amblyopia management
- Regular follow-up visits with ophthalmologist and pediatric oncologist for years to monitor for side effects and possible recurrence
- Monitor for cataract formation and amblyopia and treat promptly.
- Proton beam radiotherapy might be superior to traditional photon radiotherapy.
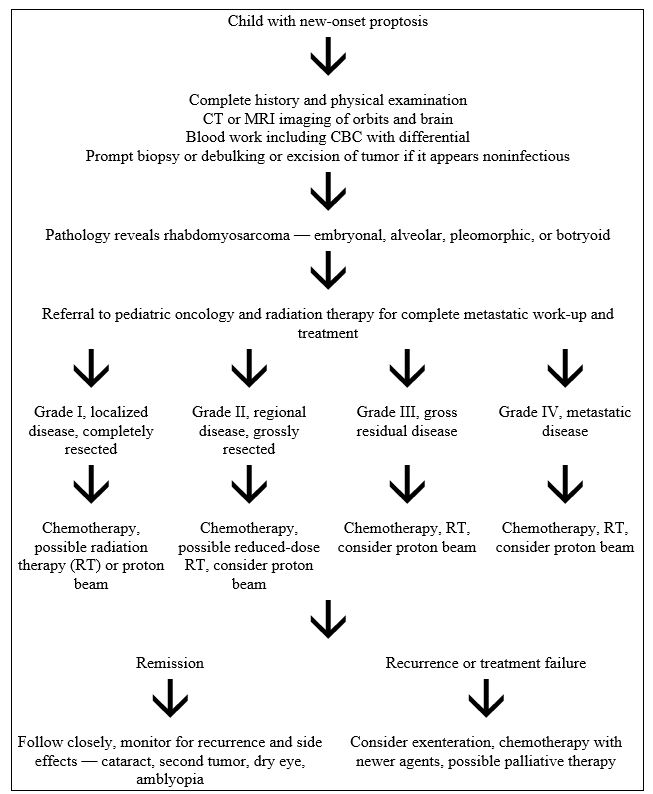
Flowchart
References and additional resources
- Arndt CA, Hawkins DS, Meyer WH, Neglia JP, Anderson JR: Comparison of results of a pilot study of alternating vincristine/doxorubicin/cyclophosphamide and etoposide/ifosfamide with IRS-IV in intermediate risk rhabdomyosarcoma: a report of the Children’s Oncology Group. Pediatr Blood Cancer 50: 33-36, 2008.
- Crist WM1, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, Breneman J, Qualman SJ, Wiener E, Wharam M, Lobe T, Webber B, Maurer HM, Donaldson SS. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 19:3091-3102, 2001.
- Crist WM, Garnsey L, Beltangady MS, et al: Prognosis in children with rhabdomyosarcoma: a report of the intergroup rhabdomyosarcoma studies I and II. Intergroup Rhabdomyosarcoma Committee. J Clin Oncol. 8: 443-452,1990.
- Crist W1, Gehan EA, Ragab AH, Dickman PS, Donaldson SS, Fryer C, Hammond D, Hays DM, Herrmann J, Heyn R, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 13:610-630, 1995.
- Friedmann AM,Tarbell NJ, Schaefer PW, et al. Case 4-2004: A nine-month-old boy with an orbital rhabdomyosarcoma. N Engl J Med 350: 494-502, 2004.
- Heyn R, Haeberlen, Newton W, et al. Second malignant neoplasms in children treated for rhabdomyosarcoma. Intergroup Rhabdomyosarcoma Study Committee. J Clin Oncol 11:262-270, 1993.
- Heyn R, Ragab A, Raney RB Jr, et al: Late effects of therapy in orbital rhabdomyosarcoma in children. A report from the Intergroup Rhabdomyosarcoma Study. Cancer 57: 1738-1743,1986.
- Hug EB, Adams J, Fitzek M, et al: Fractionated, three-dimensional, planning-assisted proton-radiation therapy for orbital rhabdomyosarcoma: a novel technique. Int J Radiat Oncol Biol Phys 47: 979-984, 2000
- Karcioglu ZA, Hadjistilianou D, Rozans M, DeFrancesco S: Orbital rhabdomyosarcoma. Cancer Control 11: 328-33, 2004.
- Kassel SH, Copenhaver R, Arean WM: Orbital rhabdomyosarcoma. Am J Ophthalmol 60: 811-818,1965.
- Khatib N, Merks JHM, Markenstein JE, et al. Long-Term Outcomes After Multidisciplinary Treatment for Pediatric Orbital Rhabdomyosarcoma. Cancers (Basel). 2025;17(4):615.
- Kodet R, Newton WA Jr, Hamoudi, AB, et al: Orbital rhabdomyosarcomas and related tumors in childhood: relationship of morphology to prognosis – an Intergroup Rhabdomyosarcoma study. Med Pediatr Oncol 29: 51-60, 1997.
- Li FP, Fraumeni JF: Rhabdomyosarcoma in children. J Nat Cancer Inst 43: 1365-73,1969.
- Mannor GE1, Rose GE, Plowman PN, Kingston J,Wright JE, Vardy SJ; Multidisciplinary management of refractory orbital rhabdomyosarcoma. Ophthalmology.104:1198-1201, 1997.
- Maurer HM, Beltangady M, Gehan, et al: The Intergroup Rhabdomyosarcoma Study –I. A final report. Cancer 61: 209-20,1988.
- Maurer HM1, Gehan EA, Beltangady M, Crist W, Dickman PS, Donaldson SS, Fryer C, Hammond D, Hays DM, Herrmann J, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer 71:1904-1922,1993.
- Newton WA Jr1, Soule EH, Hamoudi AB, Reiman HM, Shimada H, Beltangady M, Maurer H. Histopathology of childhood sarcomas, Intergroup Rhabdomyosarcoma Studies I and II: clinicopathologic correlation. J Clin Oncol. 6:67-75, 1998
- Pan M, Zhou M, Xie L, et al. Recent advances in sarcoma therapy: new agents, strategies and predictive biomarkers. J Hematol Oncol. 2024;17:124.
- Parham DM, Webber B, Holt H, et al: Immunohistochemical study of childhood rhabdomyosarcomas and related neoplasms. Results of an Intergroup Rhabdomyosarcoma study project. Cancer 67: 3072-3080,1991.
- Rao AA1, Naheedy JH, Chen JY, Robbins SL, Ramkumar HL. A clinical update and radiologic review of pediatric orbital and ocular tumors. J Oncol. 2013;2013:97590
- Ragab AH, Heyn , Tefft M, et al: Infants younger than 1 year of age with rhabdomyosarcoma. Cancer 58: 2606-2610,1986.
- Raney RB1, Anderson JR, Barr FG, Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol. 23:215-220, 2001.
- Raney RB Jr., Donaldson MH, Sutow WW, et al: Special considerations related to the primary site in rhabdomyosarcoma: experience of the Intergroup Rhabdomyosarcoma Study, 1972-76. Natl Cancer Inst Monogr 56: 69-74, 1981.
- Scupham R, Gilbert EF, Wilde J, Wiedrich TA: Immunohistochemical studies of rhabdomyosarcoma. Arch Pathol Lab Med 110: 818-821,1986.
- Shields JA, Shields CL; Rhabdomyosarcoma: review for the ophthalmologist. See comment in PubMed Commons below. Surv Ophthalmol. 48:39-57, 2003.
- Shields CL, Shields JA, Honavar SG, Demirci H; Clinical spectrum of primary ophthalmic rhabdomyosarcoma. Ophthalmology.108:2284-2292, 2001.
- Sobel RK, Ford JR, Dong W, Shriver E, Griepentrog GJ, Debnam JM, Esmaeli B. Frequency and Clinical Course of Residual Orbital Masses After Treatment of Orbital Rhabdomyosarcoma. Am J Ophthalmol. 2022;234:28–36.
- Sohaib SA, Moseley I, Wright JE: Orbital rhabdomyosarcoma – the radiological characteristics. Clinical Radiology 53: 357-362,1998.
- Tokuda PJK, Demizu Y, Wang T, et al. First report of retroperitoneal rhabdomyosarcoma treated with space-making particle therapy for preservation of renal function. Int Cancer Conf J. 2024;13(4):476–480.
- Wharam M, Beltangady M, Hays D, et al: Localized orbital rhabdomyosarcoma. An interim report of the Intergroup Rhabdomyosarcoma Study Committee. Ophthalmology 94: 251-254,1987.
- Yock T, Schneider R, Friedmann A, et al: Proton radiotherapy for orbital rhabdomyosarcoma: clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys 63:1161-1168, 2005.
Financial disclosures
Reviewers
Sana Ali: No disclosures
