Schwannoma (Neurilemoma)
Updated May 2024
Establishing the diagnosis
Etiology
- Neural crest origin
- Benign Schwann cell tumor of peripheral nerve sheath
- A peripheral nerve is composed of several bundles of axons referred to as fascicles.
- An axon is an extension of an individual nerve cell body, and each individual axon is invested by Schwann cells that provide myelination.
- In contrast, neurofibromas contain both nerve sheath and neural elements.
- Most commonly involved nerves are the first division branches of cranial nerve V, although in some cases, the specific nerve that gave rise to the tumor may not be obvious.
- Most commonly arise from the sensory nerves that supply the upper eyelid, conjunctiva, and forehead skin, namely the supraorbital, lacrimal, and supratrochlear nerve branches of cranial nerve V
- Therefore, they typically result in extraconal lesions due to the location of the nerves within the superior orbit
- In contrast to orbital schwannomas, intracranial tumors typically arise from cranial nerve VIII, or less commonly, from V, IX, and X.
- Intracranial schwannomas not associated with cranial nerves tend to occur in the first 2 decades of life and thus are considered to be developmental choristomas arising from displaced neural crest cells.
- Rarely can develop from a motor nerve such as the oculomotor, trochlear, and abducent nerves (Shen 1993)
- Also reported to rarely arise from the ciliary nerves of the choroid (Shields 1986)
Epidemiology
- Approximately 1%–2% of orbital tumors (Jakobiec, Shields 2004)
- Mainly adults age 20–50 years
- Rarely children, congenital case reports
- Slight female predominance
- Predilection for head and neck region
- Can be found in 1.5% of all patients with neurofibromatosis type 1
Pathology
- Grossly, the tumor is encapsulated with a tan appearance and can have cystic areas of mucinous degeneration as well as areas of hemorrhagic necrosis.
- Homogeneous, well-circumscribed tumor consisting of Schwann cells
- Microscopically, 2 main patterns are seen:
- Antoni-A: Hypercellular solid areas with elongated-to-oval palisading spindle cells
- Antoni-B: Hypocellular areas with stellate cells and loose mucoid stroma
- Verocay body refers to the palisading nuclei of schwannoma cells admixed within acellular zones.
- Hyalinized vessels are frequent findings.
- Antoni-B type areas are more vascular than Antoni-A areas, accounting for different signals on MRI enhancement with gadolinium.
- Antoni-A pattern correlate with hyperintensity and Antoni-B pattern correlate with hypointensity of T1-weighted MRI. (Pointdujour-Lim et al. 2018)
- Loose, lightly eosinophilic material within the cysts stain positive with alcian blue and mucicarmine, indicating the presence of mucin.
- As schwannomas age, they become more hypocellular, with a predominance of collagen and focal hyalinization, resulting in the variant known as the “ancient schwannoma.”
- Do not significantly enhance with contrast CT/MRI due to decreased vascularity
History
- Slow, progressive proptosis over years, typically painless
- Rare rapidly enlarging lesions have been described (Ma et al. 2017).
- Lid swelling or palpable anterior orbital mass
- Decreased vision due to direct optic nerve compression
- Afferent pupillary defect, constricted or generalized depression of visual fields, papilledema, or optic atrophy can be present, but can be absent even with large lesions.
- Subtle visual decline can occur very slowly over several months to years and often improves after resection.
- Cockerham (1999) described 3 patients who had VA decline to only 20/25 in 2 patients and 20/50 in 1 patient (see below for more details).
- Diplopia
- Induced hyperopia with globe indentation (choroidal folds)
- Trigeminal numbness or pain (supraorbital or supratrochlear branch) seen in less than 7% of cases
- Typically painless despite arising from cranial nerve V (Cockerham 1999)
- Increased intracranial pressure with optic nerve compromise
Clinical features
- Slow-growing tumor
- Space occupying orbital mass
- Can be intraconal or extraconal, but more often extraconal due to nerve of origin
- Calcification is unusual in orbital lesions, but cyst formation is characteristic.
- Earlier presentation typically seen in orbital apex tumors due to compressive effects on optic nerve and muscles
Testing
- CT imaging shows an enhancing, circumscribed ovoid tumor, usually without calcifications, and cysts and bony changes might be seen (Figure 1).
- Encapsulation helps differentiate lesion from a neurofibroma.
- Lesions typically located in superior orbit
- Long axis of tumor usually in anteroposterior direction, parallel to the nerve
- Bony changes such as widening of the superior orbital fissure, bowing, or contouring of bone, focal orbital expansion/fossa remodeling might be seen (“expansion without erosion”).
- Suggestive of long-standing growth process
- Bony destruction/erosion might indicate malignant etiology.
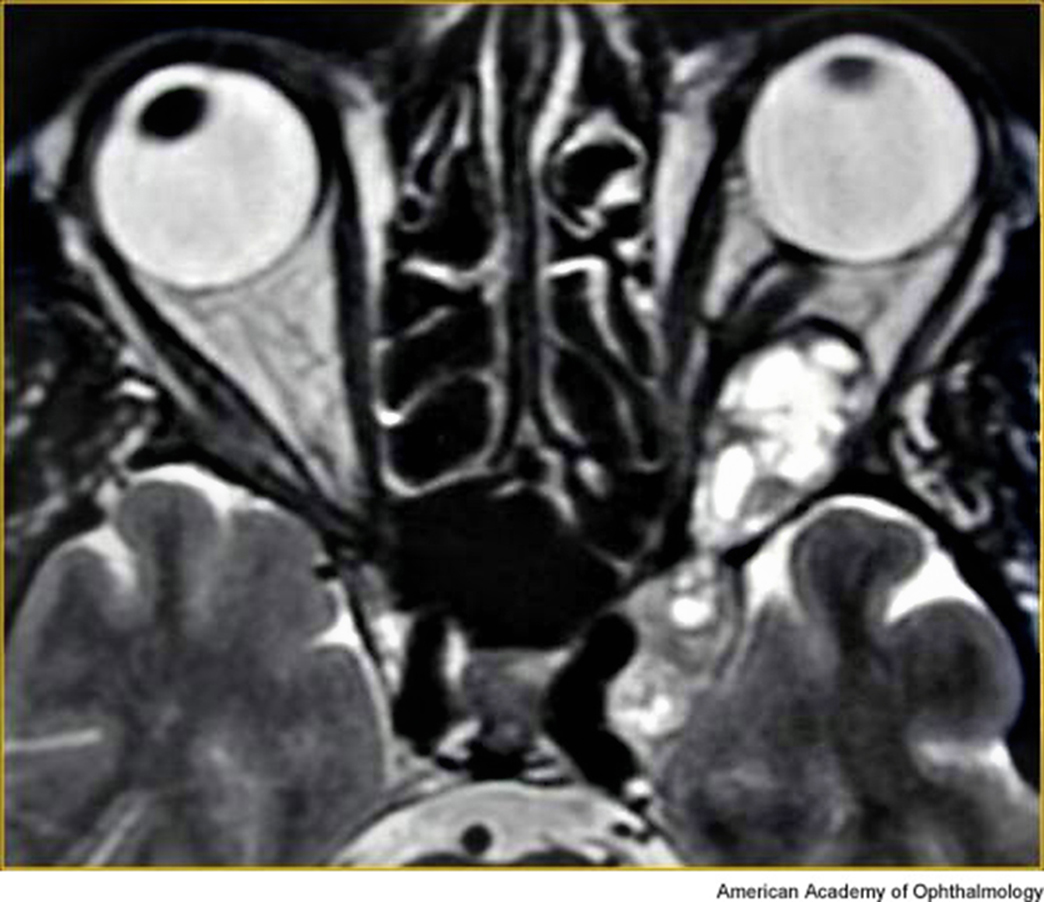
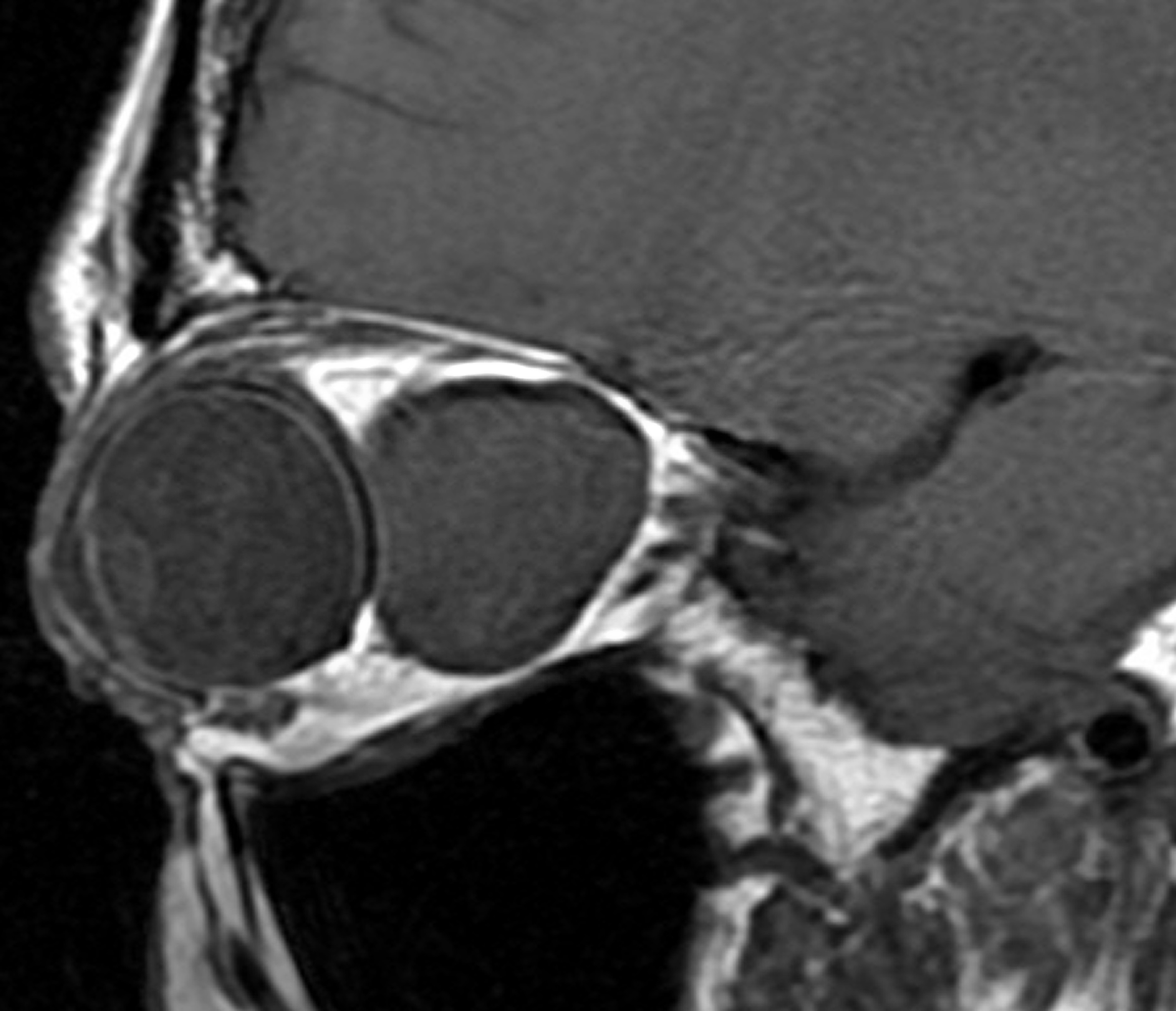
Figure 1. Typical CT image of orbital schwannoma.
- Magnetic resonance imaging (MRI) shows tumor to be isointense to extraocular muscles and brain on T1-weighted images, slightly hyperintense to muscles and brain on T2-weight images, and hypointense to orbital fat.
- Young et al. (2018) described the radiological features in patients with orbital frontal nerve schwannomas.
- 13 patients with histopathologically proven schwannomas. Mean age was 44.4 years and 61.5% were male.
- 2% were multilobulated, 30.8% were dumbbell, 15.4% were oval, and 7.7% were fusiform in shape.
- Target sign, fascicular sign, and cystic degeneration were seen in 76.9%, 35.8%, and 46.2% of patients, respectively.
- Lesion can be intraconal or extraconal, depending on the nerve involved.
- With intravenous injection of contrast, lesions demonstrate homogeneous or heterogeneous moderate to marked contrast enhancement.
- Typically increased enhancement in Antoni-B type myxoid areas in contrast to the more cellular Antoni-A areas
- Cystic schwannomas can appear as a homogeneous hypodense mass that does not enhance with contrast.
- Dynamic time-resolved MRI/MRA can be helpful to distinguish the diffuse enhancement of a schwannoma from the focal enhancement (beginning with the feeder vessel) and slow filling-in of a cavernous malformation.
- Fluid-fluid levels in orbital schwannoma have been demonstrated on MRI, presumed to be due to hemorrhage within a lesion (Gunduz 2011).
- Ultrasound of orbit
- B-scan ultrasound can demonstrate an encapsulated solid lesion containing macrocystic areas with well-demarcated borders and fairly high internal reflectivity.
- A-scan ultrasound can show echographic findings of medium to high reflectivity in Antoni-A areas, and low reflectivity in Antoni-B areas (Byrne 1988).
- Typically this is a lesion in which diagnosis is reached clinically based on history, exam, and imaging.
- Treatment options are considered based on clinical diagnosis, prior to histopathologic confirmation.
- Histopathology of excised lesion (see pathology above)
- Immunohistochemistry
- Due to neural crest origin, will stain positive for S-100 (Gunduz 2011), in contrast to meningioma
- Diffuse strong immunoreactivity for S-100 and pericellular collagen type IV almost universal in all schwannomas (Rodriguez 2012), consistent with the presence of a continuous pericellular basal lamina
- Glial fibrillary acid protein (GFAP) expressed commonly in schwannomas [reported in 15/38 (38%) cases by Kawahara 1988].
- GFAP-positive cells were usually spindle-shaped and appeared preferentially in the perivascular region of hyalinized, thick blood vessels.
- Other markers frequently positive include podoplanin (Naber 2011), calretinin (Fine 2004), and SOX10 (Nonaka 2008).
- Very rarely, otherwise typical schwannomas may show anomalous expression of cytokeratins (Fanburg-Smith 2006). Such tumors are also strongly GFAP-positive, suggesting cross reactivity of cytokeratin antibodies with GFAP, rather than true protein expression.
- Electron microscopy: Schwann cells with abundant basement membrane and extracellular long-spacing collagen (Luse bodies) can be seen.
Risk factors
- Age 20-50 years
- Slight female predilection
- Von Recklinghausen neurofibromatosis (NF) seen in 2%–18% of cases (Gunduz 2003)
- In general, most cases of orbital schwannomas are not associated with NF.
- NF can also be associated with other tumors in the differential diagnosis such as neurofibroma, optic nerve glioma, optic nerve sheath meningioma.
Differential diagnosis
Cavernous malformation
(See Cavernous malformation.)
- Common retrobulbar tumor of dilated vascular channels most often found in patients between 20-50 years old, often an incidental finding following head imaging for other reasons
- Female predilection
- MRI findings similar to schwannoma, showing isointense appearance to extraocular muscles on T1W images, and hyperintense signal on T2W images
- Can present clinically with decreased vision, axial proptosis, hyperopic shift, choroidal folds, similar to mass effect of schwannomas
- On imaging, cavernous malformations tend to display heterogeneous progressive enhancement, whereas schwannomas enhance at their maximum level immediately (Jayaram et al. 2017).
- Time-resolved MRI/MRA imaging with contrast kinetics, which uses very rapid acquisition of MRIs to provide dynamic information regarding the intravascular flow of orbital lesions, has a highly characteristic pattern for cavernous malformations.
- Demonstrate delayed contrast enhancement and limited dynamic flow within the lesion with almost imperceptible and markedly delayed blushing that might actually represent capsular enhancement
- Early feeder vessel seen, followed by only trace contrast accumulation “filling-in” within the lesion, even in the late venous acquisition frames
Optic nerve sheath meningioma
- Well-defined uniform or irregular tubular thickening or globular thickening of the optic nerve sheath meninges, which can extend from the apex into the optic canal (in contrast to schwannomas)
- Optic nerve itself appears normal in size or smaller in diameter within an area of thickened meninges, compared to the contralateral nerve at the same level.
- Smaller nerve size is due to circumferential compression or atrophy and is a useful differentiating finding as opposed to an intrinsically expanded nerve that is more commonly seen in optic nerve glioma or other inflammatory lesions.
- Calcification of the lesion is common and can have several appearances:
- Surround the nerve as a “tram-track” appearance on axial CT scan, which refers to a hyperdense linear enhancement of the meninges surrounding a hypodense nerve
- Diffuse punctate areas along the nerve
- An en-plaque signal along the optic canal, that may be difficult to distinguish from normal bone
- In general, calcification may be masked by contrast agents and should be sought on precontrast images
- Typically demonstrate a well-defined moderate to marked homogenous enhancement after intravenous contrast infusion
- Also seen in association with von Recklinghausen NF
- Advanced visual loss can be seen with even a minimal 2–4 mm of proptosis
- MRI with high (≥ 1.5 tesla) field strength, fat suppression, and gadolinium contrast demonstrate lesions to be isointense or slightly hypointense to brain and optic nerve tissue on T1-W images, and typically hyperintense (less often hypointense) on T2-W images.
- Carotid angiography might discern multiple tumor vessels and a late blush.
- Histopathology can contain psammoma bodies, which represent concentric layers of calcification.
Fibrous histiocytoma, solitary fibrous tumor
(See Fibrous Histiocytoma.)
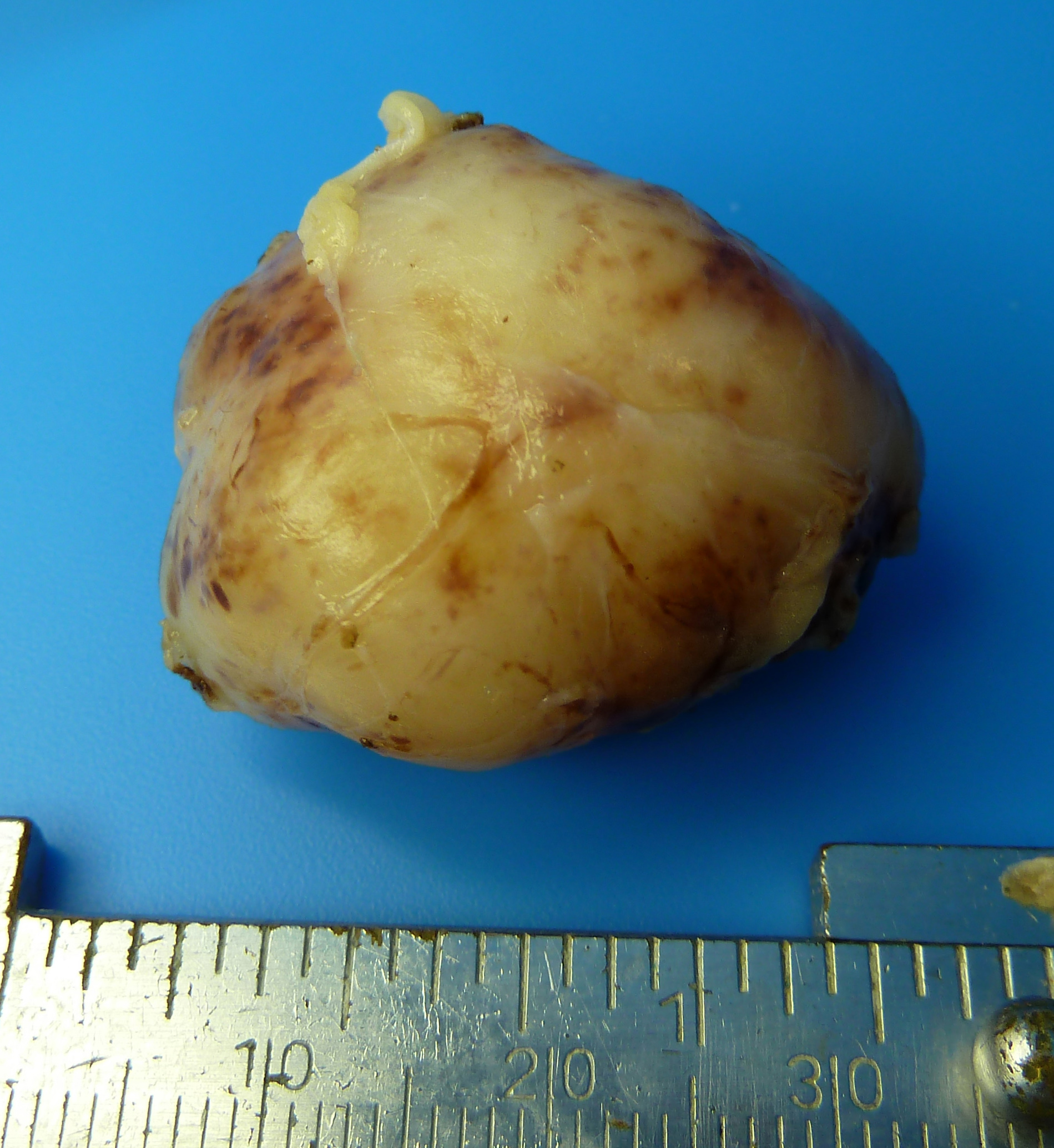
Figure 2. Gross specimen of completely excised fibrous histiocytoma.
- Consist of spindle-shaped collagen-producing fibroblasts and multinucleated histiocytes containing lipid that often demonstrate a “storiform” cartwheel pattern of tumor cells on histopathology
- Well-circumscribed, solid, round mass typically in the retrobulbar space with CT and MRI characteristics similar to schwannoma
- Commonly seen in the superior orbit, similar to schwannoma
- Moderate to marked heterogeneous enhancement with gadolinium
- Time-resolved MRA with contrast kinetics demonstrates slow delayed diffuse “filling-in” blush of lesion, highly similar to a cavernous malformation, but with perhaps slightly less blush (Warner 2013).
- Immunohistochemistry typically negative for S-100 protein and positive for CD34 and vimentin
Neurofibroma
[See Neurofibromas, Neurofibromatosis Type 1 (von Recklinghausen disease).]
- One of the more common differential diagnoses of orbital schwannomas
- Also seen in association with von Recklinghausen NF
- Unlike schwannomas, neurofibromas often present as eyelid masses infiltrating the orbit along fascial planes, and are typically circumscribed, but not encapsulated.
- A pseudocapsule can occur due to long-standing compressive effects on surrounding tissues
- Consist of localized proliferations of endoneural fibroblasts and Schwann cells arranged into fascicles, surrounded by an Alcian blue-positive myxoid matrix
- Also stains for S-100 protein, as well as demonstrates immunoreactivity for CD34 in a “fingerprint pattern”
- CT and MRI findings are similar to schwannomas, with also a predilection for being superior orbit extraconal masses.
- Slight irregularities of the border might indicate lack of true encapsulation.
- Widening of the superior orbital fissure is less commonly seen.
Hemangiopericytoma
(See Hemangiopericytoma.)
- Malignant vascular tumor that arises from the capillary pericytes and are seen most commonly in middle-aged females
- Often intraconal retrobulbar or supraorbital mass, similar to schwannomas, although margins can be less distinct due to tendency to invade adjacent tissue
- Bony erosion can be present
- MRI characteristics very similar, with isointense appearance to extraocular muscles on T1W images, and hyperintense signal on T2W images
- Marked enhancement with contrast due to vascular nature of lesion
- MRI/MRA or time-resolved contrast MRA best differentiate hemangiopericytomas due to an early brisk florid blush in the early arterial phase with dense contrast staining that is maximal on the early venous phase, as opposed to the delayed slight to moderate vascular blush in schwannomas.
- Surgical removal indicated if suspicious for hemangiopericytoma due to its malignant nature
Malignant peripheral nerve sheath tumor
(See below section.)
Orbital lymphoma
(See Lymphoma.)
- Predominantly a B-cell tumor that manifests as a homogeneous orbital mass that classically molds itself to surrounding orbital tissues, without infiltration or bony destruction
- MRI findings similar to schwannoma, showing isointense appearance to extraocular muscles on T1W images and hyperintense signal on T2W images
- Mild to moderate contrast enhancement
- Can present in various ways, but typically demonstrating a painless and insidious growth
- Complete hematologic/oncologic evaluation necessary as often will develop systemic lymphoma
Orbital metastasis
Patient management: treatment and follow-up
Natural history
- Slow-growing tumor, often managed with prolonged observation
- Tumor can grow to large sizes and extend into the orbital apex, often without decreased vision or motility deficits, thus supportive of a benign, encapsulated, very slow-growing mass
- Can be associated with von Recklinghausen’s disease, but not typical for most orbital schwannomas
- Represent approximately 1% of orbital tumors
- Malignant transformation extremely rare
- Can extend through the superior orbital fissure (rarely the inferior orbital fissure)
- Enlargement of the foramen rotundum, foramen ovale, cavernous sinus, or pterygopalatine fossa also reported
- Cellular schwannoma variant is relatively uncommon, but important to recognize.
- High cellularity, fascicular growth pattern, increased mitotic activity, and occasional locally destructive behavior, including bone erosion, often lead to consideration of malignancy.
- Composed almost entirely of a compact, fascicular proliferation of well-differentiated, cytologically bland Schwann cells, lacking Verocay bodies (Casadei 1995), and showing no more than very focal Antoni-B pattern growth (<10% of tumor area)
- Important findings include the presence of foamy histiocyte aggregates, a well-formed capsule containing lymphoid aggregates, and diffuse strong S-100 protein and pericellular collagen IV expression.
- In contrast, diffuse S-100 protein expression is extremely uncommon in spindled malignant peripheral nerve sheath tumors.
- Cytokeratin immunoreactivity can be seen in some cellular schwannomas, but might represent cross reactivity with GFAP.
- Cellular schwannomas lack expression of smooth muscle actin, desmin, CD117, and DOG1, allowing exclusion of other tumors in the differential diagnosis such as leiomyosarcoma and gastrointestinal stromal tumors.
- Despite the very high cellularity and mitotic activity, these typically lack malignant potential for practical purposes and never metastasize.
- Local recurrence is variable (5%–40%), and might be higher than in conventional schwannomas, perhaps due in part to its propensity for deep anatomic regions that are not always amenable to gross total resection.
- However, even recurrent lesions have been shown to grow slowly and do not result in death (White 1990).
- Plexiform schwannoma variant usually occurs in superficial (cutaneous or subcutaneous) locations and is defined by a plexiform (intraneural-nodular) growth pattern.
- Although might be associated with NF2 and schwannomatosis, the association is weak, seen in only about 5% of cases (Berg 2008).
- Tumor might be less circumscribed than conventional schwannoma or even lack a capsule.
- Usually composed of Antoni-A type areas
- Melanotic schwannoma variant is a rare, distinctive, potentially malignant neoplasm characterized by epithelioid cells with variably sized nuclei and marked accumulation of melanin in neoplastic cells and melanophages
- Psammoma bodies can be seen.
- Differential diagnosis must include other melanin-producing neoplasms, i.e., melanoma.
Radiation therapy
- Can be used after excision to decrease recurrence
- More recently, multisession gamma knife radiosurgery (GKRS) has been reported to be an effective primary management strategy to treat solitary, benign, well-circumscribed orbital apex tumors
- Goh et al. (2013) reported a retrospective interventional case series of 5 patients with visual disturbances from a well-circumscribed orbital apex tumor (3 cases of cavernous malformation, 2 schwannoma).
- Each patient underwent treatment with GKRS in 4 sessions with a radiation dose of 10 Gy to the tumor, and 5 Gy to the tumor margin with a prescription isodose of 50%.
- This was a cumulated radiation dose of 40 Gy to the tumor and 20 Gy to the tumor margin.
- All patients demonstrated improvement in visual acuity, pupillary responses, color vision, and visual field.
- Tumor shrinkage was observed in all patients and remained stable until the last follow-up.
- No adverse events were noted during or after radiosurgery.
- No patients experienced any radiation-related ocular morbidity.
- Multiple sessions achieved the cumulative total radiation doses needed to cause tumor shrinkage while avoiding radiation to orbital lesions of more than 8–10 Gy in a single session, which can result in injury to the anterior visual pathway.
Surgery
Surgical excision in symptomatic patients
Observation is typical primary management unless there is visual compromise to the optic nerve, corneal exposure from significant proptosis, or worsened discomfort.
Complete excision is optimal and often achievable due to its well-circumscribed, encapsulated nature.
As the tumor originates from the Schwann cells and is thus located along the periphery of the nerve, it can usually be carefully removed from the nerve with minimal trauma.
Approach governed by location
Most often a superolateral orbitotomy or upper lid crease anterior orbitotomy, with an extraperiosteal approach, is used due to the predilection of the tumor to the superior orbit.
Transfrontal craniotomy/orbitomy in conjunction with neurosurgery might be indicated for extensive superior orbit and apical lesions.
- Complications include CSF leak, intracranial injury, meningitis, postoperative ptosis, frontalis palsy.
Transcaruncular approach or transnasal endoscopic removal can be useful for medial lesions.
- Interactive image guidance systems can be useful to aid in locating the tumors intraoperatively.
Cryoprobe can be useful in extracting posterior lesions with a small dumbbell extension into the superior orbital fissure (Shields 1986).
Ideally avoid injury to vital orbital structures
If the tumor extends into the superior orbital fissure, incomplete excision might be necessary to preserve the structures traversing this space.
- Shields (1986) reported a case of a large encapsulated ovoid schwannoma involving the entire orbit and extending into the superior orbital fissure without causing motility deficits or supraorbital nerve sensory changes.
- CT demonstrated enlargement of the superior orbital fissure, and showed that the majority of the tumor was within the orbit except for a small dumbbell extension.
- Complete removal via a lateral orbitotomy with blunt dissection and cryoprobe was successful without postoperative visual or motility complications.
Orbital decompression
- Can be a therapeutic alternative for a small subgroup of tumors in the intraconal orbital apex — particularly inferior to the optic nerve — that would otherwise cause significant ocular morbidity with excision (Kloek 2006)
- Kloek et al. (2006) reported a retrospective case series of 5 patients with compressive optic neuropathy secondary to an orbital apex tumor who underwent surgical decompression.
- All patients had progressive visual decline measured by visual loss, afferent pupillary defect, decreased visual field, and in some cases, color vision deficits.
- No patients had a history of malignancy or rapid clinical decline.
- All tumors were clinically diagnosed as benign tumors (schwannoma or cavernous malformation) based on radiologic features such as well-defined margins, lack of infiltration, and homogeneous enhancement pattern.
- 3/5 patients underwent serial preoperative imaging that documented no change in tumor size.
- Surgical decompression of the orbit was via transnasal endoscopic decompression of the medial orbital wall, lateral orbitotomy, or transcaruncular decompression approaches.
- None had biopsy or resection of the tumor.
- All patients had improved visual function by 2 weeks to 6 months after surgery.
- Postoperative complications included
- Ptosis
- Enophthalmos
- Recurrent optic neuropathy
- Diplopia
Common treatment responses, follow-up strategies
- Typically good response to surgery, even if tumor is only partially excised
- Rose (1991) reported 54 cases of peripheral nerve sheath orbital tumors and found that despite incomplete resection in 24% benign schwannoma cases, there were no recurrences requiring additional surgery in 23 years of follow-up.
- Low rate of recurrence
- Close follow-up after surgery
Preventing and managing treatment complications
Risks of orbital surgery
- Bleeding, hemorrhage
- Infection
- Iatrogenic injury to surrounding structures (globe, optic nerve, muscles, nerves)
- Loss of vision
- Incomplete excision
- Cerebrospinal fluid leak from the superior orbital fissure or roof
- Damage to branches of the ophthalmic artery, causing central retinal artery occlusion (Rose 1991)
- Transient or permanent mydriasis due to injury to or impairment of blood flow to ciliary ganglion
- Postoperative ptosis or diplopia from injury to levator superioris palpebrae or superior rectus muscles
Disease-related complications
Vision loss
Cockerham (1999) described 3 patients who had subtle VA decline over several months to years, all which improved to baseline following surgical resection.
In this article, the total number of patients seen with schwannoma with or without visual decline was not mentioned.
Case 1
- A 19-year-old female presented with proptosis and decreased vision over the past several years.
- There was no diplopia, pain, or numbness.
- VA was 20/15 OD, 20/25 OS. Pupillary and motility exams were normal.
- 4 mm left proptosis and 2 mm hypoglobus were present.
- A pterional approach to remove the superomedial orbital mass extending to the superior orbital fissure was performed, with the portion in the cavernous sinus left unresected.
- The resected portion measured 42 x 13 x 12 mm.
- VA improved to 20/15 postoperatively.
Case 2
- A 53-year-old female presented with decreased vision for 3 months.
- There was no diplopia, pain, or numbness.
- VA was 20/50 OD, 20/20 OS.
- A right RAPD was present, and motility was normal.
- 2 mm right proptosis without vertical displacement was present.
- A transantral approach with ethmoidectomy was performed to resect the posterior-inferior orbital tumor.
- VA improved to 20/20 and RAPD resolved postoperatively.
Case 3
- A 23-year-old female presented with several months of throbbing frontal headaches and decreased vision starting during her second trimester of pregnancy.
- There was no diplopia or numbness.
- VA was 20/25 OU.
- Pupillary and motility exams were normal.
- 2-mm right proptosis, bilateral disc edema, macular striae, and prominent bifrontal fullness were present.
- Imaging demonstrated a large anterior cranial fossa mass extending into the ethmoid and frontal sinuses, and both superomedial orbits.
- Complete resection was achieved through a bifrontal craniotomy and sinusectomy.
- VA improved to 20/20 OU and papilledema resolved within several weeks.
In all 3 patients, a misdiagnosis or delay in diagnosis likely occurred as none had trigeminal dysfunction, which has previously been considered an important sign in schwannoma.
In Case 3, the significant headaches and papilledema were initially confused for physiologic changes during pregnancy and delivery.
Involvement of adjacent tissue
Also intracranial extension
Malignant transformation extremely rare
(Schatz 1971)
Recurrence (rare)
- As described previously, recurrences are rare and can take many years to grow.
- Recurrent tumors typically have low malignant potential.
- Can demonstrate hypercellularity without frank pleomorphism, and have increased mitotic figures on histopathology
- Complete excision should be performed.
Historical perspective
Rose (1991) presented 54 cases of orbital peripheral nerve sheath tumors and described 28% of patients with orbital neurofibromas had a family history and/or systemic signs of neurofibromatosis in contrast to 0% of the patients with benign schwannomas (P < 0.01).
Malignant peripheral nerve sheath tumor of the orbit (MPNST) is a rare tumor previously referred to as malignant schwannoma, neurogenic sarcoma, neurofibrosarcoma, and malignant neurilemoma (Wanebo 1997).
- Enzinger and Weiss (1988) suggested that the term malignant schwannoma created the false impression that these tumors arose from pre-existing benign schwannomas, an event that occurs extremely rarely.
- Although the Schwann cell and perineural fibroblast have both been implicated in the histogenesis of the malignant peripheral nerve sheath tumor, the exact cell of origin still remains controversial.
- Approximately half of MPNSTs arise de novo.
- Other MPNSTs have also been reported to occur in up to 2% to 29% of patients with neurofibromatosis type 1.
- These tumors often extend far beyond the grossly defined tumor margins; therefore, intraoperative frozen sections to delineate surgical margins is necessary if an MPNST is suspected.
- Although MPNSTs typically favor large nerve trunks, such as the brachial or lumbar plexus, they can rarely occur in the orbit.
- They have a propensity to arise from the supraorbital branch of the trigeminal nerve, thus presenting as a mass beneath the medial lid in the anterior supranasal orbit.
- Jakobiec (1985) also reported a series of eight cases of orbital MPNST, all arising from a supraorbital branch of the trigeminal nerve.
- Malignant nature of tumor is not often suspected clinically due to a relatively benign exam without skin erosion or significant periorbital inflammation.
- Often described clinically as a medial cystic mass
- No gender predilection
- Recurrences are common, occurring in 3–6 months after surgery (Jakobiec 1985).
- Can extend intracranially and metastasize, particularly to the lungs, and often within 2 years of presentation
- Histopathology is markedly hypercellular with hyperchromatic nuclei arranged in fascicles, but can be variable with heterologous elements.
- Often, densely cellular fascicles of tumor cells alternate with less densely cellular or hypocellular areas.
- In extraorbital locations, heterologous elements such as myogenic elements of rhabdomyoblastic differentiation, can be seen.
References and additional resources
- AAO, Basic and Clinical Science Course. Section 4: Ophthalmic Pathology and Intraocular Tumors, 2013-14.
- AAO, Basic and Clinical Science Course. Section 7: Orbit, Eyelid and Lacrimal, 2013-14.
- Berg JC, Scheithauer BW, Spinner RJ, et al. Plexiform schwannoma: a clinicopathologic overview with emphasis on the head and neck region. Hum Pathol 2008;39:633–40.
- Byrne BM, van Heuven WAJ, Lawton AW. Echographic characteristics of benign orbital schwannomas (neurilemomas). Am J Ophthalmol 1988;106:194-8.
- Casadei GP, Scheithauer BW, Hirose T, et al. Cellular schwannoma. A clinicopathologic, DNA flow cytometric, and proliferation marker study of 70 patients. Cancer 1995;75:1109–19.
- Cockerham KP, Cockerham GC, Stutzman R, et al. The clinical spectrum of schwannomas presenting with visual dysfunction: a clinicopathologic study of 3 cases. Surv Ophthalmol 1999;44:226-34.
- Dervin JE, Beaconsfield M, Wright JE, Moseley IF. CT findings in orbital tumors of nerve sheath origin. Clin Radiol 1989;40:475-9.
- Enzinger FM, Weiss SW. Malignant tumors of peripheral nerves. In Soft Tissue Tumors, 2nd edition. FM Enzinger, SW Weiss (Eds). St. Louis, CV. Mosby, 1988, pp 781–815.
- Fanburg-Smith JC, Majidi M, Miettinen M. Keratin expression in schwannoma; a study of 115 retroperitoneal and 22 peripheral schwannomas. Mod Pathol 2006;19:115–21.
- Fine SW, McClain SA, Li M. Immunohistochemical staining for calretinin is useful for differentiating schwannomas from neurofibromas. Am J Clin Pathol 2004;122:552–9.
- Goh AS, Kim YD, Woo KI, Lee JI. Benign orbital apex tumors treated with multisession gamma knife radiosurgery. Ophthalmology 2013;120(3):635-41.
- Gunduz K, Kurt RA, Erden E. Orbital schwannoma with fluid-fluid levels on MRI. Ophthal Plast Reconstr Surg 2011;27(3):e51-4.
- Gunduz K, Shields CL, Gunalp I, et al. Orbital schwannoma: correlation of magnetic resonance imaging and pathology findings. Graefes Arch Clin Exp Ophthalmol 2003;241:593-7.
- Itsuo T, et al. Differentiation of Cavernous Hemangioma from Schwannoma of the Orbit: A Dynamic MRI Study. AJR 2004;183:1799-1804.
- Jakobiec FA, Font RL, Iwamoto T. Diagnostic ultrastructural pathology of ophthalmic tumors. In: Jakobiec FA, ed. Ocular and Adnexal Tumors. Birmingham: Aesculapius, 1978; 359-453.
- Jakobiec FA, Font RL, Zimmerman LE. Malignant peripheral nerve sheath tumors of the orbit: A clinicopathologic study of eight cases. Trans Am Ophthalmol Soc 1985;83:332.
- Jayaram A, Cohen LM, Lissner GS, Karagianis AG. A retrospective review of cases preoperatively diagnosed by radiologic imaging as cavernous venous malformations. Orbit 2017;36(3):128-134.
- Kapur R, Mafee MF, Lamba R, et al. Orbital schwannoma and neurofibroma: role of imaging. Neuroimaging Clin N Am 2005;15(1):159-74.
- Kawahara E, Oda Y, Ooi A, Katsuda S, et al. Expression of glial fibrillary acidic protein (GFAP) in peripheral nerve sheath tumors. A comparative study of immunoreactivity of GFAP, vimentin, S-100 protein, and neurofilament in 38 schwannomas and 18 neurofibromas. Am J Surg Pathol 1988; 12(2):115-20.
- Kloek CE, Bilyk JR, Pribitkin EA, Rubin PA. Orbital decompression as an alternative management strategy for patients with benign tumors located at the orbital apex. Ophthalmology 2006;113(7):1214-9.
- Konrad EA, Thiel HJ. Schwannoma of the orbit. Ophthalmologica 1984;188(2):118-27.
- Ma KK, Callahan AB, Wang SJ, Goldman JE, Kazim M. Atypical rapidly enlarging orbital schwannoma. Ophthalmic Plast Reconstr Surg 2017;33(3S Suppl1):S111-S114.
- Naber U, Friedrich RE, Glatzel M, et al. Podoplanin and CD34 in peripheral nerve sheath tumours: focus on neurofibromatosis 1-associated atypical neurofibroma. J Neurooncol 2011;103:239–45.
- Nascimento AF, Fletcher CD. The controversial nosology of benign nerve sheath tumors: neurofilament protein staining demonstrates intratumoral axons in many sporadic schwannomas. Am J Surg Pathol 2007;31:1363–70.
- Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol 2008;32:1291–8.
- Ogawa K, Oguchi M, Yamabe H, et al. Distribution of collagen type IV in soft tissue tumors. An immunohistochemical study. Cancer 1986;58:269–77.
- Pointdujour-Lim R, Lally SE, Shields JA, Eagle RC Jr, Shields CL. Orbital schwannoma: radiographic and histopathologic correlation in 15 cases. Ophthalmic Plast Reconstr Surg 2018;34(2):162-167.
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of Peripheral Nerve Sheath Tumors: Diagnostic Overview and Update on Selected Diagnostic Problems. Acta Neuropathol 2012;123(3):295-319.
- Rootman, J. Diseases of the Orbit: A Multidisciplinary Approach, Second Edition. Lippincott Williams & Wilkins, Philadelphia PA. 2003; 250-6.
- Rose GE, Wright JE. Isolated peripheral nerve sheath tumours of the orbit. Eye 1991;5:668-73.
- Schatz H. Benign orbital neurilemmoma: Sarcomatous transformation in von Recklinghausen’s disease. Arch Ophthalmol 1971;86:268-74.
- Shen WC, Yang DY, Ho WL, et al. Neurilemmoma of the oculomotor nerve presenting as an orbital mass: MR findings. AJNR Am J Neuroradiol 1993;14(5):1253-4.
- Shields JA, Kapustiak J, Arbizo V, et al. Orbital neurilemoma with extension through the superior orbital fissure. Arch Ophthalmol 1986;104(6):871
- Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, Part 1. Ophthalmology 2004;111:997-1008.
- Stefansson K, Wolimann R, Jerkovic M. S-100 protein in soft tissue tumors derived from Schwann cells and melanocytes. Am J Pathol 1982;106:261-8.
- Wanebo JE, Malik JM, Vandenberg SR, et al. Malignant peripheral nerve sheath tumors. Cancer 1997;71:1247.
- Warner EJ, Burkat CN, Gentry LR. Orbital fibrous histiocytoma mimicking cavernous hemangioma on dynamic contrast-enhanced MRA imaging. Ophthal Plast Reconstr Surg 2013;29(1):e3-5.
- Weiss SW, Langloss JM, Enzinger FM. Value of S-100 protein in the diagnosis of soft tissue tumors with particular reference to benign and malignant Schwann cell tumors. Lab Invest 1983;49:299–308.
- White W, Shiu MH, Rosenblum MK, et al. Cellular schwannoma. A clinicopathologic study of 57 patients and 58 tumors. Cancer 1990;66:1266–75.
- Young SM, Kim YD, Jeon GS, Woo KI. Orbital frontal nerve schwannoma-distinctive radiological features. Am J Ophthalmol 2018;186:41-46.
