Spheno-Orbital Meningioma (Sphenoid Wing Meningioma)
Updated May 2024
Establishing the diagnosis
Etiology
Spheno-orbital meningiomas are benign tumors arising intracranially from the sphenoid ridge arachnoid villi cap cells with various configurations of intra-orbital extension.
The most common orbital component arises from tumor growth through the superior orbital fissure but optic canal extension is occasionally seen.
The classic spheno-orbital case involves an intracranial component in the anterior and/or middle cranial fossa and an intraorbital soft tissue component with associated hyperostosis and/or intraosseous tumor involvement of the greater wing of the sphenoid bone.
Epidemiology
Meningiomas represent up to 95% of benign intracranial tumors (Heufelder, OPRS 2009). Around 20% will involve the sphenoid wing.
They are twice as common in females and present with increasing frequency as patients approach middle age.
History
Common history is that if a slow onset, unilateral, painless, progressive proptosis in a middle-aged woman (Saeed, BJO 2011).
Pain of a diffuse nature, headache or focally is found in about 30%.
Vision loss is noted in 20%–60% (Oya, J Neurosurg 2011; Marinello, Clin Neuro Neurosurg).
With a significant intracranial component, seizures can occur.
Clinical features
Proptosis is the hallmark and presents near uniformly (Marinello, Clin Neuro Neurosurg 2013).
- About 44% will have < 4 mm proptosis and 35% > 4 mm proptosis (Oya, J Neurosurg 2011).
Optic canal involvement and associated decreased vision is found in 20–25% (Oya, J Neurosurg 2011).
Less common presentations from the orbital involvement include inferior globe dystopia, temporal fossa mass, eyelid edema, chemosis, trigeminal hypoesthesia, or diplopia.
Elevated intracranial pressure with associated optic nerve swelling is rare. Optic nerve swelling or pallor can also occur from intracanal compression.
Testing
Brain and orbit imaging by both CT and MRI
CT demonstrates reactive hyperostosis and calcifications within the lesion.
MRI delineates the intraorbital tumor and dural extension.
Tumors enhance homogenously with gadolinium, and the presence of dural enhancement (“dural tail”) helps distinguish meningioma from fibrous dysplasia.
Testing for staging, fundamental impairment
Classification
The tumor can be classified by various subtyping schema based on morphology.
- Intracranial meningiomas were defined by Cushing to have either mass-like or plaque-like growth patterns (Scarone J Neurosurg 2009).
- Guiot originally described “external” and “internal” sphenoid wing menigiomas, which is echoed by other authors (Scarone J Neurosurg 2009).
- External variety largely involves profound hyperostosis with sheet-like dural and intraorbital involvement (Figure 1).
- Internal varieties have more well-defined intradural and intraorbital mass lesions (Figure 2).
Other authors have classified the lesion by orbital involvement based on location at the apex or elsewhere in the orbit (Marinello, Clin Neuro Neurosurg 2013).
Examination
Because the lesions often involve the superior orbital fissure, orbital apex, cavernous sinus, inferior orbital fissure, and optic canal, a comprehensive orbital and neuro-ophthalmic examination is important to determine the level of impairment and surgical goals.
- Color testing, automated perimetry, and optic nerve exam for swelling or pallor
- Retinal nerve fiber layer OCT can also play a role in predicting potential recovery in patients with severe compressive neuropathy.
- Pupil exam for an APD, mydriasis and reactivity
- Exophthalmometry with a Hertel or perhaps preferably with a Naugle exophthalmometer as correction might involve a surgery that moves that lateral wall.
- Extraocular motility exam
- Trigeminal nerve exam including corneal sensation, V1 and V2 hyposthesia
- Levator function and MRD1
- Facial nerve function with particular attention to the frontalis muscle as the frontal branch is often temporarily weakened after stretch injury during a frontotemporal craniotomy.
- Periocular exam for signs of extraorbital extension
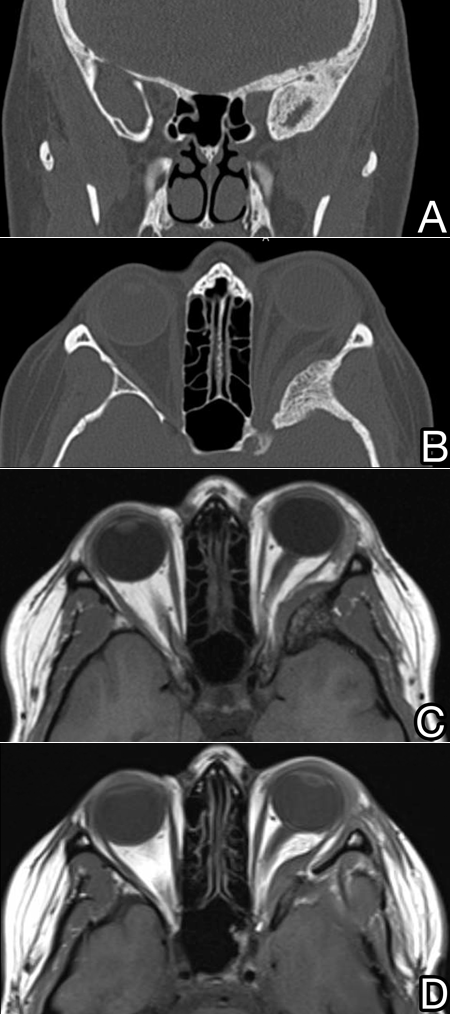
Figure 1. Patient with a “plaque-like” spheno-orbital meningioma with extensive hyperostosis around the superior orbital fissure extending throughout the skull base seen in coronal (A) and axial (B) CT images and on T1 MRI before (C) and also immediately after modified orbitozygomatic approach resection (D).
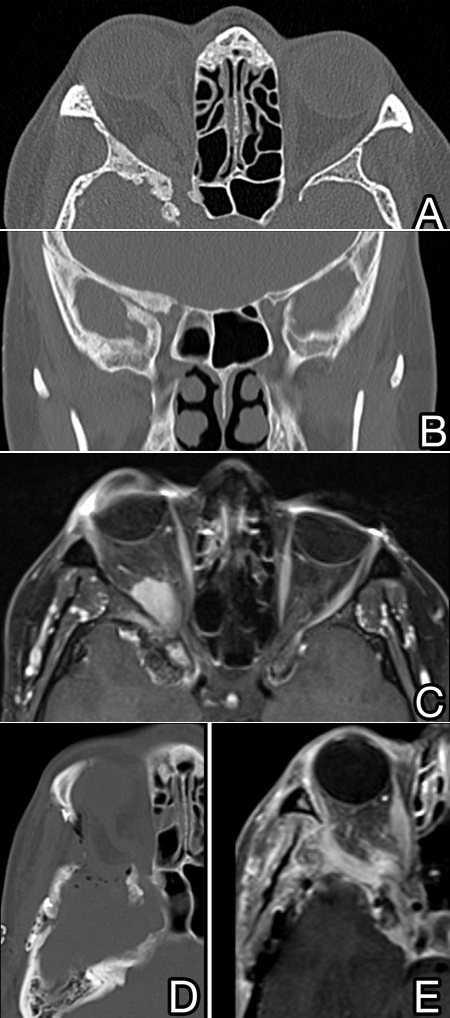
Figure 2. Patient with a “mass-like” spheno-orbital meningioma with hyperostosis and a well-defined posterior orbital mass causing rectus muscle distortion and a dural mass with temporal lobe distortion seen in axial (A) and coronal (B) CT imaging and on T1 fat-suppression MRI with gadolinium contrast (C). The patient underwent orbitozygomatic approach resection and immediate imaging was obtained postoperatively (D and E).
Risk factors
- History of neurofibromatosis-2
- Middle age, female
- History of ionizing radiation
- Growth can accelerate in pregnancy
Differential diagnosis
The differential diagnosis for proptosis is broad and includes thyroid associated orbitopathy, orbital tumor, vascular lesions, and inflammatory conditions.
Because the intraorbital appearance can be variable, various orbital tumors can appear similar radiologically, including hemangioma, neurofibroma, schwannoma, fibrous histiocytoma, hemangiopericytoma, or lymphoma.
The hyperostosis can be confused with fibrous dysplasia or uncommonly with a bone neoplasm.
Patient management: treatment and follow-up
Natural history
Proptosis progresses slowly.
When surgery is performed, preoperative symptoms have been reported for a mean of 10 months with a range of 2 months–10 years (Scarone, J Neurosurg 2009).
A review of prior scans performed for other indications (when available) might demonstrate previously subclinical lesions.
Medical therapy
Patients are often observed until bothered by significant proptosis.
In cases in which compressive optic neuropathy, motility impairment, or cerebral edema are noted, surgery should be considered.
Radiation therapy
Radiotherapy has traditionally had an important role for nonsurgical candidates.
It has become an important component as a tool postoperatively for many cases where definitive resection (e.g., in the cavernous sinus) is not achievable as recurrence is high.
Complete resection of a tumor involving the superior orbital fissure, optic canal, and /or cavernous sinus is rarely achieved.
Surgery
Although extended lateral orbitotomy through a lid-crease incision has been used, the various approaches to the supero-posterior orbit and anterior cranial fossa often involve a craniotomy.
- Complete resection (Simpson Grade I) is rarely achievable and subtotal resection (Simpson Grade II) is the norm (Scarone J Neurosurg 2009).
A frontotemporal craniotomy of some type is preferred with the orbitozygomatic approach, modified orbitozygomatic approach (Cheng, J Clin Neurosci 2009), supraorbital-pterional approach (Marinello, Clin Neuro Neurosurg 2013), and lateral orbitocranial approaches (Marinello, Clin Neuro Neurosurg 2013) (with or without bone flaps) all being in use.
- The choice of surgical approach is likely to depend on the neurosurgical team’s preference.
- In most cases, the frontotemporal approach exposes the frontal bone, temporal fossa and orbit and a craniotomy exposes the anterior and/or middle cranial fossa and, with gentle brain retraction, provides a view of the sphenoid bone posteriorly.
- The various approaches involve further access to the orbit by removal of the rim, zygoma, or lateral wall through this exposure. Figure 3 is a 3D volumetric reconstruction of a patient after orbitozygomatic approach resection of a sphenoid wing meningioma.
- As an example, a modified orbitozygomatic approach provides excellent exposure for the orbital surgeon to operate on large posterior “mass-like” lesions.
- The removal of the zygoma as in an orbitozygomatic approach is not particularly helpful for the orbital dissection.
- It is helpful for the orbital surgeon to perform the subperiosteal orbital dissection prior to the neurosurgical team creating rim osteotomies because an intact periorbita greatly aids in controlling the mass removal.
- Subperiosteal dissection should be performed to expose the ethmoidal arteries, superior orbital fissure, and inferior orbital fissure internally. Many lesions sit above or within the periorbita without true soft tissue invasion. Some tumors, however, will require intraorbital exploration.
- After neurosurgical bone flap removal and rim removal, the periorbita can be incised in the superolateral quadrant avoiding the lacrimal gland and lacrimal artery. Through this approach, the superior orbital fissure and optic canal can be carefully drilled.
- Ultrasonic bone aspirators (e.g., Sonopet, Cuso) can decrease trauma to the neural structures passing through foramen.
- Conversely, traditional burring and associated heat production can be helpful in controlling bleeding of cancellous bone.
- Ultrasonic soft tissue tools (such as Sonopet, Cavitron) can be useful to cavitate and remove masses and “plaque-like” lesions.
Orbital reconstruction of the bony defect in the posterior orbit at the time of resection is controversial.
- Clearly, meticulous skeletal reconstruction of any orbital rim or zygomatic osteotomies should be performed.
- The adhesions of the dura (or dural repair) to the orbit posteriorly make secondary repair of enophthalmos difficult, but the high rate of recurrence of tumor and proptosis makes many surgeons hesitant to attempt correction.
- At a minimum, application of a large gel foam sheet or Duragen around the opened periorbita helps prevent entrapment of orbital fat in the osteotomy closure and should be performed.
- In cases of largely bone hyperostosis, the lateral wall can be thinned with drilling and left intact.
- If placing an implant or bone graft, the material likely only needs to reconstruct the mid orbit behind the rim.
- Consider placement of a something like small pore (“smooth”) porous polyethylene coated titanium mesh, which is somewhat flexible and radiolucent, and unlikely to cause adherence to the surrounding tissues if reoperation is needed.
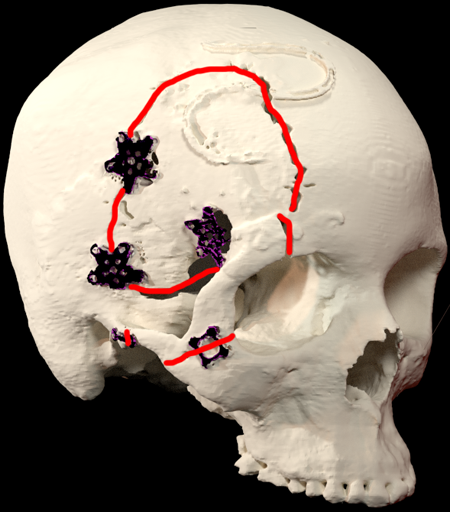
Figure 3. 3D reconstruction of the patient shown in Figure 2.
Common treatment responses, follow-up strategies
- Orbital reconstruction using deformable titanium mesh is both safe and effective.
- Visual acuity remained the same / improved in 85% of patients.
- Visual field improvement / no change in 93% of patients.
- Proptosis was reduced by a mean of 4.4 mm.
- Recurrence was noted in 20% at an average of 43 months following surgery.
Preventing and managing treatment complications
In the early postoperative period, patients often experience
- Motility deficits
- Ptosis with decreased levator function
- Frontalis weakness
- Mydriasis and numbness from either extraocular muscle or cranial nerve manipulation (Scarone, J Neurosurg 2009)
Long-term effects from surgical intervention are not rare (Oya, J Neurosurg 2011).
- Trigemenial hypoesthesia or anesthesia is noted in 3–21% of patients.
- Motility deficit attributed to cranial nerve III, IV and VI palsy is reported in 1.8%–14%.
Intraoperative stroke has been reported (Scarone, J Neurosurg 2009).
Recurrence rates of 27%–0% at 5 years have been reported for subtotal resection (Heufelder, OPRS 2009; Oya, J Neurosurg 2011).
Although CT scanning can aid in visualizing the results of bone removal, MRI performed 6 months postoperatively and yearly is the mainstay for monitoring for recurrence of the soft tissue component.
Reconstruction of the bony orbital defect is controversial.
- Lack of repair of large defects can result in pulsatile enophthalmos, meningocele or motility deficits from extraocular muscle pathway abnormalities.
Radiation can cause dry eye, optic neuritis, cataract, iritis and radiation retinopathy.
- Dose reduction is associated with fewer complications.
Disease-related complications
- Loss of vision from optic canal compression as described above
- This might not be uniformly reversible with surgery.
References and additional resources
- Cheng CM, Chang CF, Ma HI, Chiang YH, McMenomey SO, Delashaw JB, Jr. Modified orbitozygomatic craniotomy for large medial sphenoid wing meningiomas. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 2009;16:1157-60.
- Heufelder MJ, Sterker I, Trantakis C, et al. Reconstructive and ophthalmologic outcomes following resection of spheno-orbital meningiomas. Ophthalmic plastic and reconstructive surgery 2009;25:223-6.
- Mariniello G, Bonavolonta G, Tranfa F, Maiuri F. Management of the optic canal invasion and visual outcome in spheno-orbital meningiomas. Clinical neurology and neurosurgery 2013;115:1615-20.
- Oya S, Sade B, Lee JH. Sphenoorbital meningioma: surgical technique and outcome. Journal of neurosurgery 2011;114:1241-9.
- Pace ST, Koreen IV, Wilson JA, Yeatts RP. Orbital Reconstruction via Deformable Titanium Mesh Following Spheno-Orbital Meningioma Resection: Ophthalmic Presentation and Outcomes. Ophthalmic Plast Reconstr Surg. 2020 Jan/Feb;36(1):89-93.
- Saeed P, van Furth WR, Tanck M, et al. Surgical treatment of sphenoorbital meningiomas. The British journal of ophthalmology 2011;95:996-1000.
- Scarone P, Leclerq D, Heran F, Robert G. Long-term results with exophthalmos in a surgical series of 30 sphenoorbital meningiomas. Clinical article. Journal of neurosurgery 2009;111:1069-77.
